A facile, selective, high recovery system for precious metals based on complexation between melamine and cyanuric acid†
Abstract
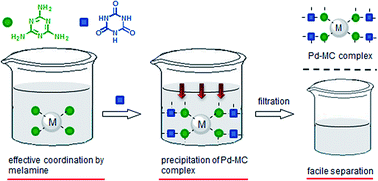
We developed a facile, selective, high recovery system for precious metals based on complexation between melamine and cyanuric acid (denoted as MC) through hydrogen bonding. The addition of melamine to an aqueous solution of metal ions allows for homogeneous coordination to metal ions with high efficiency at a high rate. After coordination, subsequent addition of cyanuric acid to the metal–melamine complexes results in precipitation of the metal–MC complex, which can be separated by filtration. The recovery of PdII using an MC system was fast and the recovery efficiency was greater than 90% within 1 min. The maximum amount of Pd recovered by the MC system (0.595 gPd/gMC) was greater than other materials in the literature. The system was also capable of selective recovery of PdII from other metal solutions. Pd recovered by the MC complex could be separated quantitatively by reductive treatment, and the MC system was recyclable at least 4 times. This system is therefore promising as a selective and high recovery system for precious metals.

 Please wait while we load your content...
Please wait while we load your content...