Hybrid amphiphilic bimodal hydrogels having mechanical and biological recognition characteristics for cardiac tissue engineering†
G. T. Finosh and
M. Jayabalan*
Sree Chitra Tirunal Institute for Medical Sciences and Technology, Polymer Science Division, BMT Wing, Thiruvananthapuram-695 012, Kerala State, India. E-mail: mjayabalan52@gmail.com; Fax: +91-471-234814; Tel: +91-471-2520212
First published on 20th April 2015
Abstract
Tissue engineering strategies rely on the favourable microniche scaffolds for 3D cell growth. For cardiac tissue engineering, a biodegradable hydrogel has to meet the essential requirements viz. adequate strength, compatibility of degradation products to the host tissue, maintenance of cellular viability and differentiation, favouring cell integration, controlled degradation of scaffold commensurate with the contractile function under ischemic conditions of the injured heart. In this work, an attempt is made to explore some of these stringent and diagonally opposite requirements. Hybrid amphiphilic bimodal hydrogels having mechanical and biological recognition characteristics were developed using graft comacromer of alginate-poly(mannitol fumarate-co-sebacate). Alginate was graft copolymerized with poly(mannitol fumarate-co-sebacate) macromer (HT-MFS). The resultant comacromer was crosslinked with PEGDA and DEGDMA to form two bimodal hydrogel scaffolds. Both hydrogels exhibited better physiochemical and mechanical properties and supported long-term cell viability under static and dynamic conditions. Laser scanning confocal microscopy Z-stacking evaluations showed infiltration of FDA-stained L929 fibroblasts in the interstices of the hydrogels with appreciable depth. The hydrogel based on PEGDA promoted cell growth to an extent of 98 μm when compared to that of DEGDMA based hydrogel with 52 μm. These hydrogels supported the co-culture of fibroblasts and cardiomyoblasts and provided a better microniche for the cells as evident by the viability and cell cycle progression analyses. The favourable cellular responses of these hydrogels are attributed to the inherent biological recognition characteristics. On comparing the two hydrogels, the PEGDA-based hydrogel was superior to its DEGDMA counterpart due to the higher hydrophilicity of the former. The PEGDA-based hydrogel is a promising candidate for cardiac tissue engineering.
1. Introduction
Tissue engineering has emerged as a promising modality to create neoorgans due to the increasing clinical demand for tissue/organ repair and replacement.1 This strategy mainly focuses on the cellular responses over the key biomimetic characteristics of the scaffolds in terms of chemical, mechanical, physical and topographical cues.2 Apart from cell–cell communication and signalling, the biochemical and physiological functions of a tissue is also regulated by the coordinated interactions between the cells and their native 3D extra cellular matrix (ECM). The understanding about the nature, composition and orientation of the ECM has opened opportunities for the ex vivo synthesis and development of biomaterial based scaffold systems mimicking ECM for improved 3D cell culture.3 Among the various types of biomaterials employed as matrices for 3D cell growth, the hydrogels subsets are more advantageous due to their high water holding capacity, potent mass transport abilities and excellent soft tissue like consistency.4–6Although the basic constituents of ECM viz. water, proteins (collagens) and polysaccharides (glycosaminoglycans) are same, the amount and types of these components vary from tissues to tissues. For example, the amount and type of collagen found in the lung tissue is different from that of bone tissue. Therefore the physical and mechanical properties vary with different tissues. For the rational designing of a hydrogel, several factors like cell growth, migration, colonization and ECM synthesis which are influenced by the hydrogel have to be considered. Hydrogels should also possess appreciable physiochemical and mechanical properties, appropriate degradation profile and a favourable morphology with respect to the native ECM of interest.7 Furthermore, the fate of the cells lies on their communication and signalling mediated through their surroundings. In order to replicate the tissue functions in vitro tissue engineering, the bioactive and signalling factors as well as and homo and hetero-typical cell interactions have to be considered; this remains as a major challenge. Still the hydrogels form one of the ideal choices for tissue engineering applications among the various classes of biomaterials.8 The 3D microenvironment provided by the hydrogels for the cell culture is advantageous for the in vitro tissue engineering as the cultures in 2D are not the exact replica of the in vivo cell growth. The cells cultured in 2D failed to express certain tissue specific genes at comparable levels as in vivo.9
Cardiovascular diseases (CVD), especially myocardial infarction (MI), contribute more than 30% of the global mortality.10 According to American Heart association, on every 25 seconds one man will have a coronary event and a death/min due to the same.11 Apart from conventional treatment modalities, the regeneration of infarcted heart muscle is a challenge due to the absence of cell division in cardiomyocytes. Cardiac transplantation is also difficult due to the dearth of transplantable heart.12 Cardiac tissue engineering approach provides a pleasant hope to the millions of CVD sufferers throughout the globe. A promising mode for cardiac tissue engineering is the combination of biocompatible hydrogel scaffolds with cells to form a cardiac tissue construct (CTC) and its implantation to the infarction site.13–15 For the success of the implantation, the CTC should mimic the structural and functional aspects of the native myocardium in terms of biomechanical properties.
The major limitations of the hydrogel scaffolds for the cardiac tissue engineering arise due to their toxicity risks, immunogenicity, improper degradation, inappropriate mechanical performance (especially under hydrodynamic conditions) and so on.15 We addressed these issues by hybridizing a novel mechanically stable synthetic polyester with a biocompatible natural polymer to form a biosynthetic hydrogel. Alginate, the natural polysaccharide from brown sea algae, has been widely used for tissue engineering due to its excellent biomimetic characters. The alginate can be easily crosslinked ionically by divalent ions like Ca2+. Nevertheless, these ionic crosslinks will be replaced with monovalent ions, which are abundant in the biological fluids, leading to the loosening of their mechanical strength.16 Moreover, unmodified sodium alginates do not promote cell attachment as reported elsewhere.17
Sebacic acid is a biocompatible linear dicarboxylic acid formed as an intermediate in mammalian system due to the ω-oxidation of long-chain aliphatic acids.18 Sebacic acid based polymeric elastomers have been used for several biomedical applications. Kim et al. reported the potential application of PEG–sebacic acid diacrylate for orthopedic applications.19 Vilaeti et al. used the same polymer for preventing the left ventricular remodelling in post infarcted rat models.20 Poly(glycerol sebacate) polymers were reported to be better for cardiovascular applications due to its biomechanical compatibility.21 Poly(glycerol sebacate) was used as stem cell delivery vehicles for cardiac regeneration.22 Poly(polyol sebacate) polymers (based on hexose sugar alcohol and pentose sugar alcohol) have been studied for biomedical applications.23 But the cytotoxicity of the acidic degradation products and fast degradation kinetics limited their use in long-term tissue engineering applications.24 Mannitol is a well known hexose sugar alcohol for its antioxidant properties. Since mannitol forms a normal metabolite in animals, it is more suitable for tissue engineering.25
The availability and abundance of surface functional groups on the scaffolds influence the attachment of cells and biomolecules. It was reported that the negative charge of the scaffold matrix enhances the cell attachment and growth.26 Thevenot et al. reported that the negative charges facilitate the binding of cell adhesion proteins like fibronectin and albumin (therefore contribute to biocompatibility too).27 Similarly the abundance of the functional groups like –OH, –COOH and –SO3H especially –OH groups were reported to enhance fibronectin mediated cell attachment and spreading.28 Despite the advantages with natural and synthetic polymeric materials, the immunogenicity and purification issues associated with the former and the deficiency in biological recognition associated with the latter limit their use as successful tissue engineering scaffolds. Being anionic the alginate enhances the negative charge density of the scaffolds. For better cell responses and mechanical compliance, we have developed biosynthetic hybrid hydrogels using comacromer of alginate and synthetic unsaturated polyester poly(mannitol fumarate-co-sebacate) prepared from the monomers sebacic acid, mannitol and maleic anhydride.
The incorporation of alginate fraction by acid catalysis to the unsaturated polyester as described in this paper can increase the availability of free –OH and –COOH. The mannitol and sebacic acid units of the polyester fraction can also contribute –OH and –COOH respectively for the cell–hydrogel interaction. These functional groups can act as cues for the adhesion and proliferation of different cell types mediated through the adsorption of adhesion molecules.
The present hydrogels were mechanically favourable and able to sustain the 3D viability of different cells when cultured alone and together under static and hydrodynamic conditions even in the absence of cell adhesion molecules and growth factors. The present paper deals with the biological performance of these novel hydrogels as potential scaffolds.
2. Results and discussions
2.1. Synthesis and physiochemical characterizations of poly(mannitol fumarate-co-sebacate)-alginate
The principal challenge in cardiac tissue engineering is the inability to produce the engineered tissue construct with long durability and delayed cell response. The improper biodegradation of synthetic biomaterials and inappropriate mechanical properties of natural polymers resulted in the decrease in viability of several cardiac scaffolds.29 The scaffolds intended for cardiac applications must possess the potential to induce proper physio-chemical stimuli and cellular biochemical and rheological responses.30 It should be non-thrombogenic and non-immunogenic.31 To overcome these issues we synthesized hybrid hydrogels using MFS comacromer and the natural polysaccharide alginate.The outstanding properties of alginate in terms of biocompatibility, biodegradability, non-immunogenicity, non-thrombogenity and chelating power pave way to use it for versatile biomedical applications, especially for cardiac tissue engineering. The immunogenicity of alginate arises due to the protein contaminants which is present in minor amounts, bound with alginate chains. Heating with the acid causes the hydrolysis of alginate to lower molecular weight fractions. While the proteins are denatured under acidic pH. The acid treatment also induces hydrolysis of bound protein contaminants in the alginate and ends up with mixture of the amino acids. In the present synthesis, sulfuric acid used as a catalyst for the condensation of alginate and the polyester enables hydrolytic removal of the bound protein contaminants and hydrolysis of alginate. In addition extensive washing enabled removal of residual contaminants. The chelation of alginates with divalent ions offers a simple way to form alginate hydrogels.32 However, the alginate-based hydrogels are mechanically weak leading to structural and morphological deformities of the scaffolds. So it fails to be a functional template for tissue regeneration.33 This mechanical instability hinders the use of alginate hydrogels for long term and load bearing applications like cardiac tissue engineering. These demerits of alginate can be resolved by grafting it with synthetic polymers. Synthetic polyesters have displayed better response for tissue engineering applications due to their controlled degradation and sustenance of mechanical properties.34 But the use of these biodegradable polyesters in soft tissue engineering is limited due to elastic deformation, acidic degradation products, absence of cell recognition signals and so on.35 To address these issues we synthesized the novel polyester MFS utilizing three cell friendly biomolecules, sebacic acid, fumarate and mannitol, for grafting with alginate.
Sebacic acid, the C10 dicarboxylic acid, is a natural metabolite formed by β-oxidation of long chain carboxylic acids and ω-oxidation of medium and short chain fatty acids. Since sebacic acid undergo cellular metabolism to form the TCA intermediate succinate, its use in cardiac tissue engineering hydrogels offers a safer application.36,37 Mannitol is the sugar alcohol formed from the hexose sugar mannose and is commonly used as food ingredients.38 In biological system, mannose will be derived mostly from glucose by a non-enzymatic process called Lobry de Bruyn–Alberda–van Ekenstein transformation.39 Mannitol can enter glycolytic pathway by the formation mannose by mannitol dehydrogenase enzyme, which can then form either glucose or fructose. Fumaric acid, the TCA intermediate, based polyesters were already proven biocompatible and non-toxic for tissue engineering applications.40 It is obvious that the degradation products of the prepared MFS polyester will not evoke any adverse response for cardiac tissue engineering when used alone.
Several reports deal with the tedious procedures for the synthesis of porous scaffolds of sebacic acid. Gao et al. reported the synthesis of poly(glycerol sebacate) by salt fusion and polymer curing technique.6 They could generate 3D microporous tissue engineering scaffolds that promoted the attachment and proliferation of several types of cells.6 However, the hydrogels synthesized by this technique faced the difficulties like poor geometric tolerance and low yield.41 Kemppainen et al. utilized solid free-form fabrication technique for the synthesis of poly(glycerol sebacate) elastomers for cartilage tissue engineering.42 Martin Frydrych reported extensive porosity in poly(glycerol sebacate) by freeze-drying technique.43 We have adopted simple condensation reactions followed by freeze-drying for the synthesis of our comacromer hydrogel scaffolds that are superior in terms of synthesis and fabrication.
Hydroxyl terminated polyester macromer; MFS was synthesized by the condensation reaction of sebacic acid, mannitol and maleic anhydride (Fig. 1). In order to enhance the reactivity the maleic acid group of the oligoester was isomerized to fumaric acid groups in the ester by vacuum condensation at high temperature. Of the six –OH groups of mannitol four were left unreacted and two were allowed to esterify with one of the –COOH groups of the dicarboxylic acids sebacic acid and fumaric acid. The OH/COOH ratio of the polyester was set at 2 so as to maintain the reactivity and amphiphilicity. Then the comacromer MFSA was synthesized by simple acid catalyzed condensation of the primary alcoholic group of mannitol in MFS and carboxylic acid group of alginate. GPC analysis of MFSA showed number average molecular weight of 849 and a weight average molecular weight of 1022. The polydispersity value 1.2 revealed the effective condensation and uniformity molecular size. From the relative lower molecular weight values (in the oligomeric range) it was clear that short alginate segments were hydrolysed from the sodium alginate and these segments were then condensed with the MFS polyester. This increases the availability of free functional groups for the cells to interact. The double bonds present in the MFSA comacromer were utilized for free radical induced crosslinking with vinyl monomers, DEGDMA and PEGDA, to form MFSA-DEGDMA and MFSA-PEGDA bimodal hydrogel scaffolds respectively. In addition, the alginate fraction of the MFSA was crosslinked with Ca2+.
The IR spectral analysis showed the characteristic peaks for the surface functional groups of the scaffolds and the effective formation of the polymer and comacromer (Fig. 2). FT-IR spectrum of MFS polymer reveals a broad band at ∼3400 cm−1 for hydroxyl group imparted by the mannitol residue. The peak at 2800 cm−1 and 2900 cm−1 are due to C–H stretching vibration of the double bond ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH of fumarate groups and also to the symmetric stretching vibration of the aliphatic CH2 groups sebacic acid and mannitol residue. The peak around 1640 is due to C
CH of fumarate groups and also to the symmetric stretching vibration of the aliphatic CH2 groups sebacic acid and mannitol residue. The peak around 1640 is due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of fumarate groups. Peaks around 770 cm−1 indicates the C–H bending of cis –CH
C stretching vibration of fumarate groups. Peaks around 770 cm−1 indicates the C–H bending of cis –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– end groups. The peaks around 1700 cm−1 revealed the carbonyl stretch indicating the ester bond formation. ATR spectrum of MFSA showed the broad spectrum of –OH groups of alginate and mannitol around 3300 cm−1. The peaks appearing at ∼1600 and ∼1400 cm−1 are due to the asymmetric and symmetric stretching vibrations of the carboxyl groups. The sharp peak appearing at 1020 cm−1 is due to the C–O–C stretching of alginate. ATR-IR spectrum of MFSA-DEGDMA and MFSA-PEGDA showed peaks around 1720 cm−1 for C–O stretching. The peaks appearing at 1600 and 1400 cm−1 are the asymmetric and symmetric stretching vibrations of the carboxylate group of alginate and due to C
CH– end groups. The peaks around 1700 cm−1 revealed the carbonyl stretch indicating the ester bond formation. ATR spectrum of MFSA showed the broad spectrum of –OH groups of alginate and mannitol around 3300 cm−1. The peaks appearing at ∼1600 and ∼1400 cm−1 are due to the asymmetric and symmetric stretching vibrations of the carboxyl groups. The sharp peak appearing at 1020 cm−1 is due to the C–O–C stretching of alginate. ATR-IR spectrum of MFSA-DEGDMA and MFSA-PEGDA showed peaks around 1720 cm−1 for C–O stretching. The peaks appearing at 1600 and 1400 cm−1 are the asymmetric and symmetric stretching vibrations of the carboxylate group of alginate and due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of unsaturated fumarate groups of MFS. The peak at 1030 cm−1 (C–O–C stretching) for alginate is an evidence for the presence of MFS and alginate on the surface of both the hydrogels.
C stretching vibration of unsaturated fumarate groups of MFS. The peak at 1030 cm−1 (C–O–C stretching) for alginate is an evidence for the presence of MFS and alginate on the surface of both the hydrogels.
The surface properties of the tissue engineering hydrogels play a crucial role in cell growth and function. The surface hydrophilicity and wettability of the MFSA based hydrogels were determined by water contact angle measurements. Both the advancing and receding angles of both the hydrogels showed values around 55 degrees revealing their amphiphilic nature (Table 1). The contact angle values can be correlated with the biocompatibility of the hydrogels. It was reported that the hydrogels with lower contact angles (high wettability) have appreciable hemocompatibility and vice versa. The materials with lower contact angle values exhibit better cell adhesion, spreading and proliferation. The surface hydrophilicity enhances plasma protein adsorption especially that of albumin, which in turn activates cell adhesion and improves the compatibility of the material.44 Wei et al. reported that cell attachment was greater in hydrogels having contact angle values around 60 degrees which is similar to our results.45 But Shu et al. reported that extreme hydrophilicity of poly anionic hydrogels based on the hyaluronic acid hinders the cell attachment and spreading.46 Our hydrogels are amphiphilic and this amphiphilicity can be attributed to the greater amount of free –OH groups contributed by alginate fraction and mannitol fractions and the hydrophobic tails of sebacic acid fractions of MFSA comacromer.
| Analyses | Parameters (n = 6) | MFSA-DEGDMA | MFSA-PEGDA |
|---|---|---|---|
| Physical | Advancing contact angle (deg.) | 57.21 ± 4.29 | 55.33 ± 5.95 |
| Receding contact angle (deg.) | 58.15 ± 3.83 | 56.16 ± 5 | |
| Swelling (%) (P < 0.001) | 241.49 ± 41.35 | 400.5 ± 28.5 | |
| EWC (P < 0.001) | 70.34 ± 3.52 | 79.95 ± 1.14 | |
| Freezable water content (%) | 26.95 | 34.64 | |
| Non frozen water content (Wnf) (%) | 43.39 | 45.31 | |
| Pore length (μm) (n = 20) (unstretched) (P < 0.001) | 5.06 ± 0.73 | 7.50 ± 1.95 | |
| Thermal | Onset of melting temp. of frozen water (°C) | −1.4 | −2.08 |
| Enthalpy of melting of frozen water (J g−1) | 90.23 | 117.1 | |
| Onset of crystallization of frozen water (°C) | −9.17 | −8.92 | |
| Enthalpy of crystallization of frozen water (J g−1) | 89.5 | 115.7 | |
| Mechanical evaluations | Tensile strength (kPa) (P < 0.001) | 266 ± 23 | 525 ± 60 |
| Elongation at break (%) (P < 0.001) | 24.16 ± 4.14 | 53.29 ± 6.46 | |
| Young's modulus (kPa) | 2455 ± 599 | 2213 ± 464 | |
| Fatigue life (no.: of cycles) | >600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>730![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Biological evaluations | Hemolysis assay | 3.71 ± 0.04 | 3.57 ± 1.2 |
| MTT assay (P < 0.01) | 98.97 ± 13.4 | 97.6 ± 9.36 | |
| Collagen estimation (% increase in OD) (P < 0.01) | 50.28 ± 3.6 | 31.51 ± 5.16 |
There was a considerable difference obtained in the EWC and swelling of the MFSA hydrogel system with MFSA-PEGDA gained more water holding capacity than MFSA-DEGDMA (Table 1). This is due to the higher hydrophilicity imparted by PEGDA cross linker to MFSA-PEGDA. On the other hand, in MFSA-DEGDMA, the DEGDMA is a short moiety and its methyl groups impart a partial hydrophobic nature when compared with PEGDA. Scaffold properties like biocompatibility, permeability, protein adsorption and mechanical strength depend on the water holding capacity of the hydrogels. The high water content, soft consistency, low interfacial tension and channelling porosity makes hydrogel an ideal substitute for native ECM.47 The water content permits the diffusion of oxygen and nutrients to the cells growing in the interstices.48
Patel et al. developed an amphiphilic polymer by introducing PEG segments to poly(glycerol sebacate) for tissue engineering applications.49 The poly(glycerol sebacate)-co-PEG block copolymer exhibited a contact angle value around 66 degrees when 60% PEG was incorporated. But it's swelling properties and water holding capacity was lower (EWC ∼ 32%).49 Kafouris et al. also reported amphiphilic poly(glycerol sebacate) elastomers with similar properties.50 The introduction of mannitol and alginate fraction along with cross linkers PEGDA and DEGDMA in our hydrogel scaffolds imparted excellent swelling and water holding capacity. This has also attributed the shifting of amphiphilicity more towards hydrophilic range than the reported poly(glycerol sebacate)s.
The ESEM images of the MFSA hydrogel scaffolds displayed their characteristic morphology, which can be utilized by the invading cells for penetration by availing the metabolites from the medium circulating through the pores. A significant difference in pore morphology was observed between MFSA-PEGDA and MFSA-DEGDMA hydrogels (Fig. 3, Table 1). This difference was induced due to the characteristic physiochemical behavior of the cross linkers. Freeze-dried MFSA-PEGDA hydrogels possessed considerably higher pore length than MFSA-DEGDMA. PEGDA is highly hydrophilic than DEGDMA and these differences will reflect in their cross linking interactions contributing to the difference in pore morphology. Moreover, the pore morphology and porosity also depend on the fabrication process.51 We employed the simple freeze-drying technique for the fabrication of both the hydrogels with uniform pore size.
 | ||
| Fig. 3 ESEM images of MFSA-DEGDMA (A) and MFSA-PEGDA (B) hydrogels showing their surface morphology. Yellow lines indicate the pores and their lengths selected for calculating average pore size. | ||
The porosity and pore size of the hydrogels play a direct and significant role in the functionality of these hydrogels for their biomedical applications. This is very vital for the cell nutrification and guides the cell proliferation, infiltration and the formation of neo tissue. In addition, the pores facilitate mechanical interlocking with the host tissue at the implant site and partially enhance the mechanical stability of the implant.52 Rnjak-Kovacina et al. reported that the scaffolds with average pore length of 11 μm supported cell infiltration and 3D growth. And scaffolds with average pore length of 8 μm allowed the cell growth on the surface only. In addition, the cell penetration in the latter was limited.53 Interestingly, both our hydrogel scaffolds had pore length below 8 μm; both hydrogel scaffolds promoted cell infiltration largely. From cardiac tissue engineering point of view, the interconnected pores with lower pore length is suitable for rapid vasculogenesis with negligible fibrosis. Such pores also aid in the synchronization and propagation of the electrical signals for contraction of cardiomyocytes with excellent mechanical compilation.54 The pore length and morphology of our scaffolds revealed its potential for cardiac tissue engineering. Still other parameters have to be considered.
The DSC analysis of MFSA hydrogel samples displayed exothermic peak due to crystallization of freezing water and endothermic peak due to the melting of frozen water. The sharp endothermic peaks, 1.83 °C for MFSA-DEGDMA and (−) 0.38 °C for MFSA-PEGDA hydrogels, appeared due to the melting of frozen water (Wf) (Fig. 4, Table 1). The frozen water is the sum of frozen free (Wff) water and frozen bound (slightly structured water) (Wfb). It was reported that the appearance of endothermic peaks around 0–10 °C for hydrogels is an indication of the frozen free and bound water.55 Our recent article also described the determination of frozen water content (Wf) from the enthalpy of melting endotherm and enthalpy of melting of pure water and EWC of the hydrogels.56 The frozen water content was higher for MFSA-PEGDA than the other hydrogel but the non-frozen bound water content was almost equal (Table 1). This will be due to the increased affinity of bulk water molecules towards the hydrogel generated because of hydrophilicity of the cross linked PEGDA segment. The freezing free water is similar to the bulk water that facilitate the easy diffusion of nutrients, metabolites and waste materials to and from the hydrogel interstices. The cells seeded onto the hydrogels can take advantage of this phenomenon. The non-freezing bound water will be able to cause the de-solvation of biomolecules and mediate the effective adsorption/desorption which in turn is crucial for cell penetration and function.57 The water content and status of both our hydrogels provide a suitable microniche for the homing of different cell types of cardiac tissue implying its cardiac tissue engineering applications.
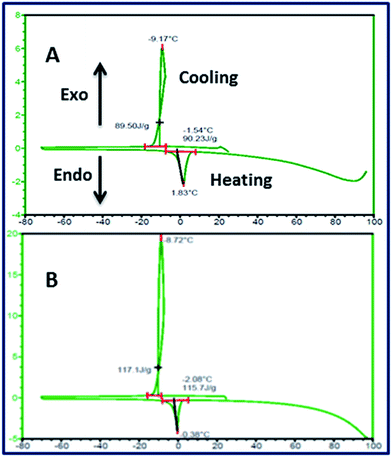 | ||
| Fig. 4 DSC thermogram of water swollen MFSA-DEGDMA (A) and MFSA-PEGDA (B) hydrogels showing the characteristic peaks for crystallization and melting of water present in the hydrogels. | ||
2.2. Mechanical evaluations of MFSA hydrogels
During cardiac cycle the heart muscle exhibit contrasting mechanical events. According to Starling relationship, in the systole it becomes ductile while in diastole it will be more elastomeric. Therefore, the mechanical properties of the cardiac tissue engineering hydrogel scaffolds are very crucial.58 Both of our hydrogels possessed appreciable mechanical properties to be applicable for cardiac tissue engineering (Table 1). The tensile strength of water swelled MFSA-PEGDA was found to be higher than that of MFSA-DEGDMA. This may be due to the presence of ample hydrogen bonding in MFSA-PEGDA imparted by more hydrophilic PEGDA segments. The hydrogen bonding partners will interact with the functional groups, especially –OH, within the hydrogel and from the surrounding medium. However, these types of interactions are minimal in MFSA-DEGDMA due to the less hydrophilic nature of the DEGDMA segment. The fatigue life cycles also displayed a similar result. Still the Young's moduli of both hydrogels fall in the same range.There are reports supporting the use of hydrogel materials for cardiac tissue engineering applications as these biomaterials can provide mechanical support to the diseased myocardium until the native myocardial ECM is restored.59 And a functional cardiac construct must possess a tensile strength greater than the mammalian left ventricular myocardium (0.15 MPa for canine models).60 The elastic modulus of the one of the most successfully used material, Dacron, was reported to be around 600 kPa. Our hydrogels have values greater than the reported values signifying their mechanical compatibility for cardiac applications. Since our materials are hydrogels it can efficiently promote cardiac tissue orchestration as it was already reported that the cardiomyocytes and associated cells prefer a softer microenvironment for growth and function.61
2.3. Studies on biodegradation
The stability of both the MFSA hydrogels were tested in the cell culture medium (DMEM) and the physiological buffer PBS. Both the hydrogels were found to be stable in DMEM for more than 1 week and there was no deformity in the size and shape was observed. Then these scaffolds were checked for their stability in PBS for a period of 4 weeks. The dry weights of the scaffolds were found to be reduced progressively when checked at an interval of 7 days. The degradation profiles of the two hydrogels were closely similar Fig. 5. The pH was dropped to slightly acidic. This indicated the cleavage of ester bonds and the formation of acidic groups. In vivo conditions, these slightly acidic products can be easily removed and buffered by the blood-buffer systems. Increase in total dissolved solids and conductivity signifies the formation of degradation products.Sebacic acid is a natural intermediate of the medium and long chain α-carboxylic acid metabolism. So the sebacic acid monomer released as with the MFSA hydrogel degradation can be effectively utilized by the body. It will be converted to acetyl (CoA) and succinyl CoA. The former is one of the key factors in TCA cycle and is involved in cholesterol biosynthesis and several other biochemical reactions. Succinyl CoA is also a key component of TCA cycle and serves as component for porphyrin biosynthesis.62 Similarly the other possible products, mannitol can enter the glycolytic pathways and fumaric acid is a TCA intermediate. Alginate fraction of the MFSA comacromer will be released due to the replacement of Ca2+ with monovalent ions form the medium. Even though alginate does not undergo any enzymatic degradation, it will be cleared very slowly from the implant site. Still alginate forms a potent biomaterial for cardiac tissue engineering applications due to its excellent biocompatibility. It is an FDA approved polymer.63 The degradation profile of both the hydrogels shows its safer use in cardiac tissue engineering applications.
2.4. In vitro assessment of RBC integrity upon contact with MFSA hydrogel scaffolds
The hydrogels were tested for their hemocompatibility by RBC aggregation assay and hemolytic assay. The hemolytic potential of both the hydrogels were found to be negligible and was within the acceptable limit of 5% (Table 1). The microscopic analysis of the RBCs treated with hydrogel extracts were failed to aggregate revealing their hemocompatibility (Fig. 6). The hemolysis assay is based on RBC lysis and the release of hemoglobin to the surrounding medium. Haemolysis analysis is one of the key tests recommended by ISO for implantable biomaterials as the particles leaching from the materials or the degradation products can interfere with host circulatory system.64 RBC aggregation, leading to rouleaux formation, is mainly depends on the composition and concentration of the medium where it suspends. This interferes with the local shear forces encountered by native RBCs and contribute the non-Newtonian behaviour of blood (a condition in which decreased blood viscosity and increased shear rate). As a result, the rouleaux will arrest and hold more RBCs and disturbs the normal rheology of the blood.65 The lack of rouleaux formation and haemolysis is a sign of absence of toxic degradation products revealing the compatibility of MFSA hydrogels to RBC integrity and blood flow.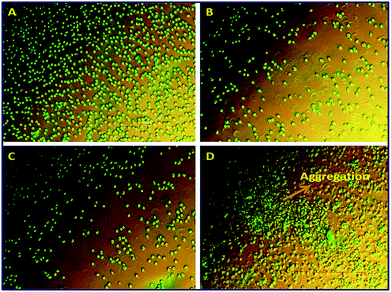 | ||
| Fig. 6 Phase contrast microscopic images showing the absence of aggregation of RBC in MFSA-DEGDMA (B) and MFSA-PEGDA (C) on comparison with +ve (D) and −ve controls (A). | ||
2.5. Studies on platelet adhesion
The thrombogenic potential of the MFSA hydrogels was evaluated by ESEM analysis after incubating with PRP (Fig. 7). The images showed no platelet adhesion to the hydrogel surface revealing the blood compatibility of the hydrogels. The platelets can adhere even on the surface of biocompatible biomaterials. However, the morphology of the adsorbed platelets determines their thrombogenic potential. If the adhered platelets retain their normal discoid morphology on the biomaterial surface they can be considered as normal and they cannot evoke thromogenesis. On the other hand, if the platelets exhibit some pseudopodia like extension, it is an indication of their activation and subsequent thrombosis.66 The ESEM analysis of both the hydrogels revealed minimal platelet adhesion and morphological changes. From the platelet adhesion studies and hemocompatibility assays, it can be concluded that the MFSA hydrogels do not evoke any adverse blood responses when implanted. | ||
| Fig. 7 ESEM images of the PRP agitated MFSA-DEGDMA (A) and MFSA-PEGDA (B) hydrogels showing absence of platelet adhesion. | ||
2.6. Assessment of cytocompatibility
The cytotoxicity of the hydrogels was determined by MTT assay and direct contact assay using L929 fibroblasts. MTT assay gives the percentage of viable cells by evaluating the metabolic activity of the cells by utilizing mitochondrial succinate dehydrogenese enzyme. The MFSA hydrogels were incubated with culture medium for 48 h and L929 cells were grown on this medium for another 48 h. As per the assay both MFSA-PEGDA and MFSA-DEGDMA hydrogels displayed viability more than 95% (Table 1) indicating the absence of the release of any toxic particles from the hydrogels. The absence of biologically harmful materials in the hydrogel extracts and greater viability is an indication of cytocompatibility of our hydrogels.67 In order to observe the real time morphologies of the cells upon contact with the hydrogels, the direct contact assay was carried out. The phase contrast images of the L929 cells grown with the hydrogels displayed no changes in their normal morphology when compared with the control. Again, the direct contact assay results also signify that the contact of cells with materials do not alter the normal physiological or biochemical characteristics of the cells. This can be manipulated for the prediction of cellular responses like adhesion, spreading, proliferation, contact guidance and so on for cardiac tissue engineering.68 Klouda et al. reported that more than 75% viability is necessary for a material to be non-toxic by MTT assay using their acrylamide-based hydrogels.69 However, both the hydrogels exhibited more than 95% viability revealing their excellent cytocompatibility. Both MTT assay and direct contact assay results signified the cytocompatibility of our hydrogels.The fluorescent microscopic analysis after live/dead staining using AO/EtBr cocktail displayed green fluorescence of AO dye indicating the retention of nuclear integrity of the cells grown on both the hydrogels (Fig. 8). This nuclear integrity is the sign of healthy and proliferating cells implying the cytocompatibility of the hydrogels and their ability to support cell growth and function. Moreover, this is the indication of the absence of apoptosis, anoikis and necrosis. Since AO is a permeable dye, it can enter all the cells and makes the nuclei to fluoresce green. While EtBr can be taken up by the cells whose membrane integrity is lost, the ability of the membrane to exclude EtBr is very much limited, and stains the nucleus red. So the health status of the cells can be evaluated by analyzing the coloration of nuclei from green to red.70 In cardiac tissue engineering point of view, the attachments of cell types to hydrogel scaffolds are very crucial. Since hydrogels form an ECM substitute, the chances of anoikis is much higher than compared to actual in vivo situations. If the attachment of the cells in the substratum (native ECM or scaffold) is improper the apoptotic pathways is triggered leading to anoikis. In normal cellular physiology, anoikis is relevant for homeostasis and tissue development by preventing the detached cells from colonizing in wrong locations. However, anoikis resistance will lead to tumor genesis and metastasis, especially while dealing stem cells for cardiac and other tissue engineering applications.71 Since both MFSA-PEGDA and MFSA-DEGDMA hydrogels provided better cell attachment, viability and proliferation without apoptosis/anoikis, they can be potent candidates for cardiac tissue engineering applications. Therefore, they can provide adherence and support to stem cells and prevents teratoma formation.
2.7. Evaluations of extra cellular responses on MFSA hydrogels
The collagen content deposited by the fibroblasts on MFSA hydrogels were determined by Sirius red assay and the OD was used to compare the collagen content. Collagen and its isoforms are the major component of the native ECM and play a significant role in tissue repair. A 50% increase in OD was observed for MFSA-DEGDMA and only 31% for MFSA-PEGDA hydrogels (Table 1). In vivo conditions, the amount of collagen deposition is balanced by the equilibrium between its synthesis and catabolism. During tissue remodelling process, the equilibrium is shifted towards synthesis and more amount of collagen is released by the fibroblasts.72 In the case of myocardial infarction, the collagen deposition leads to fibrosis and has role in the development of congestive heart failure in post infarcted heart. Moreover excessive collagen deposition impairs the morphological and functional integration of cardiomyocytes. Also this inhibits the electrical coupling and oxygen transport of the heart. Excess collagen deposition increases the stiffness of the heart muscle and the subsequent scar tissue formation affects the normal pumping function.73 For cardiac tissue engineering hydrogels, there should be a basal level of collagen deposition that is needed for the better integration of different cell types. The increase in OD on the hydrogel samples may be due to the increased surface area of the hydrogels imparted by the porosity. Therefore, cell number will be more and as a result, more collagen deposition occurs. The control used for the comparison was the cells grown on 2D culture plates. The cells were found to be grown over confluent even after 5 days in the culture plates and clustered. However, in the case of MFSA hydrogels the 3D porous environment provided extra rooms for the homing of the cells, which yielded more collagen deposits compared to the control. The present study mainly aims at the viability of these scaffolds for the in vitro tissue engineering of heart tissue. Being the major component of native cardiac ECM, the estimation of collagen itself give a clear picture about the influence of hydrogel on ECM synthesis as suggested by Frantz et al.74 However, further investigation on the expression of ECM components75 like, elastin, proteoglycans and glycosaminoglycans by the fibroblasts grown on in vitro hydrogel may yield comprehensive outcome to unravel the ECM synthesis related to cardiac tissues.The SDS-PAGE analysis of the plasma proteins adsorbed on the MFSA hydrogels showed a thick band corresponding to that of albumin (Fig. 9). From this, it was very vivid that the major fraction of the protein adsorbed on the hydrogels was serum albumin. Immediately after implantation plasma protein adsorption onto the biomaterial surface occurs. Even in the case of in vitro cell culture on the hydrogels, the same events take place and the initial interaction of cells occurs on the adsorbed proteins and not with the hydrogels. The response of the cells on the hydrogels therefore mainly depends on the adsorbed protein layer. Of the three major plasma proteins, the predominant one, the plasma albumin is reported to have a passivation effect on the surface of the biomaterials and so it would resist the acute inflammatory reactions thereby contributing to the biocompatibility of the material.76
 | ||
| Fig. 9 SDS-PAGE analysis of the plasma proteins after adsorption on the hydrogels – albumin standard (A), control (2), MFSA-DEGDMA (C) and MFSA-PEGDA (D). | ||
The amount and the type of protein adsorption and their responses are mediated by the surface properties of the hydrogels like chemistry, charge, roughness and so on. The wettability and water content of the hydrogels is another key factor for the adsorption of desired proteins like albumin. There are reports that the albumin adsorption is favored by amphiphilic surfaces rather than hydrophobic or hydrophilic. The hydrophobicity results in the denaturation of native conformation and hydrophilicity inhibits adsorption due to the formation of undesirable hydrogen bonds.77 Contact angle measurements signify the amphiphilic character of our hydrogels. Reports on the computer simulation studies revealed that water molecules would compete with fibrinogen for binding the hydroxyl groups of the hydrogel surfaces. This binding is very tight but the optimum concentration of –OH group favors albumin over fibrinogen. Still fibrinogen can interact hydrophobically to the methyl-functionalized surfaces. Carboxyl (–COOH) groups also inhibit fibrinogen binding, as it prefer water molecules than the fibrinogen protein.27 Our hydrogels bear ample –OH groups contributed by alginate and mannitol and sufficient –COOH groups by alginate and sebacic acid segments. Moreover, the freezing free and freezing bound water provide abundant water molecules for competition. Even though MFSA-DEGDMA hydrogels carry methyl groups, it will be masked by the hydrophilic functional groups and water content. The relative absence of fibrinogen adsorption and extensive albumin passivation on both the MFSA hydrogels provide better cell response and excellent biocompatibility.
2.8. Evaluations of cellular responses on MFSA hydrogels
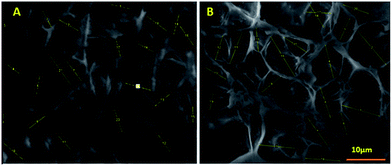
The fibroblast cell infiltration on the MFSA hydrogels was quantified for 30 days by MTT assay. Even though both the hydrogel displayed appreciable cell penetration, the extent of penetration was higher in MFSA-PEGDA hydrogel than that of MFSA-DEGDMA. MFSA-DEGDMA hydrogels maintained around 60% viability after 30 days while MFSA-PEGDA exhibited more than 90% viability (Fig. 10).Laser scanning confocal microscopy Z-stacking evaluations showed infiltration of FDA stained L929 fibroblasts towards the interstices of the hydrogels for appreciable depth (Fig. 11, ESI video 1 and 2†). The analysis revealed that the MFSA-PEGDA hydrogels supported better cell ingrowth (upto 98 μm) when compared to that of MFSA-DEGDMA (upto 52 μm). The results were in accordance with the cell infiltration assay. From these evaluations it was very vivid that the MFSA-DEGDMA hydrogels supported cell attachment and growth at the surface while MFSA-PEGDA home the cells on both the surface and interiors.
This is due to the increased pore size of MFSA-PEGDA hydrogels that favour increased infiltration than the DEGDMA counterpart. The porosity has much relevance in directing the cells to the inner networks of the hydrogels for enhancing the tissue formation by assuring a homogeneous cell distribution. The increased porosity and optimum pore size favours cell infiltration by promoting the easy diffusion of gases and metabolites towards the interior of the scaffolds that guide the cells to migrate. Upon implantation, the porosity favours angiogenesis to occur and this can be indirectly correlated with the extent of cell infiltration. More cell infiltration reflects the availability of inner pores for cell homing and this supports neo-angiogenesis.78 A recent report says that a minimum of 5 μm pores are necessary for vascular ingrowths and 5–15 μm for fibroblast infiltration.79 The pore length of the present hydrogels lies within this limit revealing their potential to promote cell infiltration. Fan et al. reported that their double network hydrogels showed viability around 90% after 21 days of seeding. Both of our hydrogels exhibited a better viability of 100% after 21 days and MFSA-PEGDA hydrogel retained around 95% viability even after 30 days.80 The water content, amphiphilicity, albumin passivation, biocompatibility and long-term cell infiltration and survival make our hydrogels an excellent choice for long-term cardiac tissue engineering applications.
Since our hydrogels are intended for cardiac tissue engineering applications, we have assessed cardiomyoblast cell attachment on MFSA hydrogels by FDA staining. The results showed better cell attachment and spreading of the cells on both the hydrogels. The cell density was found to be uniform on both the hydrogels (Fig. 11). The prerequisite for an ideal tissue engineering scaffold is its ability to support cell adhesion, migration, proliferation and function. The cell adhesion is based on the interactions between the membrane receptors and specific ligands on the supporting substratum.81 In actual conditions, these interactions are mediated by mechanical, physical and biochemical factors.82 In the case of bare hydrogels (not functionalized with cell adhesion molecules and signals), the cell adhesion occurs via weak chemical bindings like hydrogen bonding, electrostatic, polar or ionic interactions between cell membrane molecules and various functional groups of the hydrogel matrix. For this type of attachment, there will not be any involvement of native ECM proteins or components. If the hydrogel scaffolds are efficiently biocompatible to support cell adhesion and growth, the cells synthesize, secrete and deposit ECM components in a relatively short period of 24–48 h after initial seeding and start to grow on their own ECM components. The failure to do so leads to apoptosis/anoikis.83 The cell infiltration studies revealed that both our hydrogels were able to promote long-term cell viability even in the absence of cell adhesion molecules.
2.9. Assessment of cardiomyoblasts proliferation and viability by direct contact flow cytometry
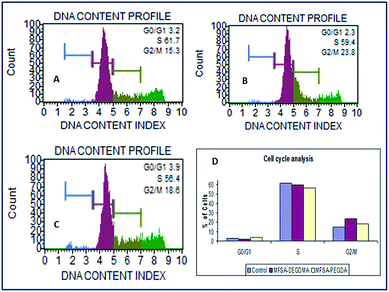
The cell count and viability was determined by flow cytometry analysis. As per the assay the viability of cells grown in contact with the MFSA hydrogels were found to be 85.8%, 86.6% and 90.2% respectively for control, MFSA-DEGDMA and MFSA-PEGDA. Both the hydrogels possessed viability greater than the control (without scaffolds). From the results, it was very clear that there was no perturbation induced to the cells due to the presence of MFSA hydrogels. The flow cytometry assay revealed the compatibility. The major problems we faced during the flow cytometry analysis of cells grown on MFSA hydrogels were the presence of hydrogel debris and subsequent clogging of the aspirating capillary tube of the instrument. Moreover the trypsinization of cells from the hydrogels and their washing and spinning resulted in the loss of cell populations. In order to get rid of these difficulties we have introduced the direct contact flow cytometry assay for hydrogel systems as this procedure is simple and it will be able to avoid the clogging of the capillary tubes by the hydrogel debris. Since the cells are in direct contact with the hydrogels, any toxicity exhibited by the hydrogels reflects in the viability and health status of the cells. Therefore, this can be extrapolated to the actual cell environment.The cell cycle analysis of H9c2 cells grown on MFSA hydrogels showed around 60% of the cells in the G0/G1 phase of the cell cycle (Fig. 12). These cells were actively proliferating and functionally healthy similar to the control. The cell population with greater DNA content is an indication of healthy cells without apoptosis or necrosis.84 The flow cytometric technique for cell cycle determination depends on the quantification of the DNA content in the cells using DNA specific dyes. The emitted fluorescence is proportional to the DNA content of the cells.85 The normal cell cycle involves G1 and G2 phases where the cells prepare itself for DNA replication and mitosis by synthesizing protein and RNA synthesis. The S phase involves the DNA replication and in M phase cytokinesis occurs. G0 is another phase where the cells become inactive and exist in a quiescent stage. The time taken for cell division and the relative lengths of the phases vary with cell type and growth conditions.86 The cell cycle analysis of MFSA hydrogels revealed that the cells are constantly progressing through all the phases of the cell cycle as in the case of control.
 | ||
| Fig. 12 FDA stained images of H9c2 cells adhered on the MFSA-DEGDMA (A) and MFSA-PEGDA (B) hydrogels. | ||
2.10. Co-culture of fibroblasts and cardiomyoblasts
In order to study the effect of one type of cells on the growth and viability of other types we co-cultured L929 fibroblasts with H9c2 cardiomyoblast cells on our hydrogel scaffolds. The viability was determined by MTT assay. The excessive proliferation of fibroblasts was controlled by arresting their cell cycle by mitomycin treatment. However, these fibroblasts were able to execute their ECM secretory function and acted as a feeder layer for cardiomyoblast cells. The OD values of the cardiomyoblast cells co-cultured with cell cycle arrested fibroblasts were found to be increased significantly on MFSA-PEGDA hydrogels when compared with the scaffold less controls. Similar results were obtained for MFSA-DEGDMA hydrogels too. However, the increase in OD values was minimal when compared with scaffold less controls. The results were shown in Fig. 13. It has been reported that the co-cultured cells may secrete nutrients, trophic factors or cell adhesion molecules, which benefit all the cell types to perform their tissue functions in the tissue engineering scaffolds.87 The presence L929 cells on our hydrogels did not affected much on the viability of H9c2 cells, even if the cells were from different source. This showed that both the cell types grow independently by depending on the secreted molecules from the both. Lu et al. reported the co-culture of mouse fibroblasts with human corneal cells and claimed that mouse fibroblast feeder layer was far better than explant culture. The report highlights the fact that in actual application the cells have to be replaced with the cells from the species of interest and has to be expanded ex vivo before implantation.88 Hussain et al. conducted a similar study like ours using chitosan scaffolds for cardiac tissue engineering by co-culturing rat cardiomyocytes and mouse fibroblasts.89 The present MFSA hydrogels also promote the growth of different cells of cardiac tissue indicating the potency for cardiac tissue engineering applications.2.11. Growth and adhesion of cardiomyoblasts under hydrodynamic condition
The growth of H9c2 cells under hydrodynamic conditions were monitored using the dynamic rotary cell culture system (RCCS) for 10 days. The viability assays showed viability greater than 50% after 10 days (Fig. 14). The AO stained images showed the cluster of cells homed on the pores of both the hydrogels. The size of the cell clusters were found to be increased on the 10th day when compared with that of 5th day (Fig. 15). This indicates the effective communication among the cardiomyoblast cells on both the scaffolds to get clustered and to function as a single unit. During the course of time, these clusters cover the entire scaffold and provide better integration of the cells. Since our hydrogels are biodegradable, the degradation of the scaffolds will leave a cell mass, which might be able to integrate with the host myocardium after implantation. However, more studies and validations are needed to extend this aspect to the clinical arena. Cell growth and response on most tissue engineering scaffolds were studied in static culture conditions where the cell attachment mainly depends on the cell density; the cell attachment cannot be further increased beyond an optimum level. In hydrodynamic conditions, the adherence of the cells to the scaffolds occurs because of the fluid flow created by the rotary culture system. Here the cell agglomerate and establishes better communications while attaching to the scaffolds and improves the spatial distribution of cells in the scaffolds. The dynamic fluid flow enhances cell proliferation, differentiation, migration and function. Moreover, the dynamic system modulates the shear properties of a native tissue environment especially dealing with cardiac tissue engineering hydrogel scaffolds.90 McDevitt reported that around 109 functional cardiomyocytes were needed to replenish the damaged ventricles after infarction.91 The 3D culture on hydrogels was found to be effective for the replication of physiochemical and structural components of the heart.92 Still the hydrodynamic conditions of the cells growing on a 3D hydrogel scaffold also influence the success of the application. In order to address this issue we cultured H9c2 cardiomyoblast cells in rotary cell culture system [(RCCS, High-Aspect Rotating Vessel model) (HARV)] as the rotation motion provide a homogenous distribution of cells and nutrients. The RCCS will also minimize the shear stress and provide an appreciable microniche for the cell culture.93. | ||
| Fig. 14 Co-culture of H9c2 cardiomyoblast and L929 fibroblast cells on MFSA-DEGDMA and MFSA-PEGDA hydrogels. L929(M) indicate mitomycin treated L929 cells. | ||
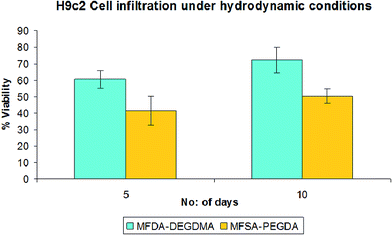 | ||
| Fig. 15 H9c2 cardiomyoblast cell infiltration on MFSA-DEGDMA and MFSA-PEGDA under hydrodynamic conditions. | ||
Synthetic polymers like expanded Polytetrafluoroethylene, commercially called as Gore-tex®, and Polyethylene terephthalate (PET), marketed by Maquet Cardiovascular under the trade name Dacron® and polyurethanes (PU) have been widely used clinically for cardiac applications due to their mechanical compatibility and biological responses. But the higher chances of immune activation and thrombosis offer a hurdle for the long term use of these materials. Apart from these, the uncontrolled oxidation and subsequent degradation limits the application of PU for cardiac tissue engineering.94 Similarly the application of natural biomaterials like hyaluronic acid, fibrin and collagen possess certain disadvantages like poor cell and protein adsorption, thrombosis and mechanical insufficiency that limits their cardiac applications.95 Decellularized materials like Matrix P/Matrix Plus® were also reported to possess disadvantages like stenosis, pseudoaneurysm and inflammation.96 Functionalization of these materials with several specific biological cues improves the performance to a greater extent. The MFSA-PEGDA and MFSA-DEGDMA hydrogels were synthesized using highly compatible biomolecules and the possible degradation products can enter energy metabolism. The extension of the in vitro responses of MFSA based hydrogels to post infarcted in vivo models can address much of the demerits associated with the conventional cardiac tissue engineering biomaterials and more studies are recommended for in vivo aspects (Fig. 16).
 | ||
| Fig. 16 AO stained images of cells grown on MFSA-DEGDMA [5th (A) and 10th (C) days] and MFSA-PEGDA [5th (B) and 10th (D) days] hydrogels. | ||
The present MFSA hydrogels were proven superior to the poly sebacate scaffolds in terms of water content, amphiphilicity, mechanical properties, compatibility and cardiac specific responses. Moreover, we have not introduced any functionalization onto our hydrogels to attract cell population in terms of cell adhesion, signalling molecules or growth factors. Still both our bimodal hydrogels have inherent biological recognition characteristics, which promote long-term viability even under hydrodynamic conditions with fibroblasts and cardiomyoblasts. Both the cell types were able to grown together in the hydrogels without any hindrance. This made our bimodal hybrid MFSA hydrogels a potent candidature for cardiac tissue engineering.
3. Experimental
3.1. Materials
Sodium alginate [medium viscosity, product no. A2033, guluronic acid (39%) and mannuronic acid (61%)] from brown algae, sodium chloride, maleic anhydride, acrylic acid, calcium chloride, diethylene glycol dimethacrylate, poly (ethylene glycol) diacrylate, L-ascorbic acid, ammonium per sulfate, etc. were obtained from Sigma Aldrich, Spruce Street, St. Louis, USA. Sodium acetate, morpholine, sodium hydroxide, DMSO etc. were supplied by Merck specialities Pvt. Ltd, Mumbai, India. Mannitol, sebacic acid etc. were purchased from HiMedia Laboratories Pvt. Ltd, India.3.2. Synthesis of poly(mannitol fumarate-co-sebacate)-alginate based hydrogel scaffolds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to get the comacromer. The comacromer was coded as MFSA.
10 to get the comacromer. The comacromer was coded as MFSA.Determination of molecular weight. The molecular weight of HT-PMFS and MFSA was determined by gel permeation chromatography. 50 μl of 0.1% solution of the resin in tetrahydrofuran (THF) was injected to the HPLC system (M/S Waters Corporation, USA) connected with 600 series pump and 2414 refractive index detector. The styragel columns (HR-5E/4E/2/0.5) were connected in series. THF was pumped at a flow rate of 1 ml min−1. The relative calibration was done with polystyrene standards (Mp – 100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 9130, and 162).
000, 9130, and 162).
FT-IR spectroscopy. FTIR analysis of HT-MFS and MFSA comacromer was carried out using FT-IR impact 410-spectrophotometer (M/S SpectraLab Scientific Inc. Canada) as per the standard ASTM E 1252-94. The spectrum was recorded using a resin smear on the KBr window.
Physiochemical characterizations. The surface functional group analysis of freeze-dried MFSA-PEGDA and MFSA-DEGDMA hydrogels was carried out using ATR spectral analysis. The surface hydrophilicity was evaluated by contact angle measurements employing Wilhelmy method using KSV sigma 701 tensiometer (M/S KSV Instruments Ltd, USA). The EWC (Equlibrium Water Content) and % swelling of both the hydrogels were determined from the dry weight and wet weight. The surface morphology of MFSA-PEGDA and MFSA-DEGDMA hydrogels was investigated by environmental scanning electron microscopy (ESEM) (FEI, Quanta-200, USA). The average pore diameter of scaffolds was calculated using the imaging software ImageJ 1.46r. The details of these evaluations were described in our previous article.56
Thermal analysis for the evaluation of water transition state. The status of water present in the hydrogels were investigated with differential scanning calorimetry (DSC) (M/S TA Instruments Inc., USA) as per ASTM 537-07 method. The freezing water content of the hydrogels was calculated from the enthalpy of melting from the DSC thermogram as described in our previous work.97
Mechanical evaluation. Water swollen MFSA hydrogels were tested for their tensile properties using the universal automated mechanical test analyzer (Instron, model 3345, M/S Instron India Pvt Ltd, Chennai, India). The samples were tested with a load cell of 100 N at 25 °C with a crosshead speed of 10 mm min−1. The data were calculated and recorded using Bluehill software.
The ability of water-swollen MFSA based hydrogels (n = 6, 4 cm × 1.5 cm) to withstand cyclic stretching were determined using the biomaterial testing instrument connected to a biobath chamber and associated temperature controller (M/S Test Resources, Model-BioBath, USA). The test was carried out in water at physiological temperature at a frequency of 6 cycles per s and amplitude 2.5 mm using the load 0.91 N. The fatigue life cycles of the hydrogels were recorded by the desktop computer using the MTL-Windows-v7.1 software.
Assessment of hemocompatibility. The hemocompatibility of both the hydrogels were determined by the assessment of hemolysis, RBC aggregation and thrombocyte adhesion studies. Fresh human blood from healthy donors with informed consent was chosen for the evaluations. The details of the procedures are described in our recent article.56
Assessment of cytocompatibility. The murine fibroblast cell line, L929 was selected for in vitro cytocompatibility studies. The cells were cultured in basic medium composed of Dulbecco's Modified Eagle's Medium with high glucose (Invitrogen), supplemented with 10% fetal bovine serum (FBS), antibiotic–antimycotic (Gibco) and sodium bicarbonate. The cells were maintained at 37 °C at 5% CO2 in a humidified atmosphere (Thermo Fischer Scientific, Forma II, USA). The medium was replaced with fresh medium once in 3 days. The cells were split after attaining around 70–80% confluence.
The cytocompatibility of MFSA-PEGDA and MFSA-DEGDMA hydrogels were evaluated by the determination of cell viability on hydrogel extracts by MTT assay, growth of cells on direct contact with L929 cells and the anti-apoptotic effects of cells grown on hydrogels by live/dead assay using acridine orange/ethidium bromide cocktail.56
3.3. Evaluations of extra cellular responses on MFSA hydrogels for cardiac tissue engineering applications
3.4. Evaluations of cellular responses of MFSA hydrogels
Determination of infiltration and long term viability. L929 fibroblasts were grown on both the MFSA hydrogels for a period of 1 month under standard cell culture conditions. Media was changed once in 3 days and the cell infiltration was quantified by MTT assay on every week. The cell-grown scaffolds were taken out from the wells, washed twice with PBS and incubated for 3 h with 1 ml MTT solution (1 mg ml−1 in PBS). After incubation, the formazan crystals formed inside the cells within the scaffolds were extracted with isopropanol containing 0.01 N HCl, vortexed for 10 min, kept for 30 min, centrifuged at 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min to settle the scaffold and cell debris. The OD of the supernatant was read at 570 nm. A control wells without scaffolds and blank containing scaffolds without cells were also maintained in the same manner. From the OD values, the percentage viability was calculated as per our previous article.56
000g for 5 min to settle the scaffold and cell debris. The OD of the supernatant was read at 570 nm. A control wells without scaffolds and blank containing scaffolds without cells were also maintained in the same manner. From the OD values, the percentage viability was calculated as per our previous article.56
Quantification of infiltration depth by laser scanning confocal microscopy. L929 fibroblasts were allowed to grow on both the hydrogels for five days. After five days the cells were washed thrice with PBS and then stained with the fluorescene diacetate. The stained hydrogel samples were again washed twice with PBS and immediately viewed under a laser scanning confocal microscope (Carl Zeiss LSM). The images were captured at 20× objective (Epiplan-Neofluar 20×/0.5 HD) with a pinhole 296 μm using the beam splitters MBS:HFT 405/488 using the excitation wavelengths 488 nm through the filter CHS1:507-539 employing Z-stack scanning mode. The cell infiltration depth was quantified by choosing specified scanning depth (2–4 μm for each stack). The images so obtained were evaluated using Zeiss LSM image examiner software.
Cell count and viability. The count and viability of the cells grown with the MFSA hydrogel scaffolds were determined by using Muse™ Count and Viability assay kit. To a subconfluent monolayer of H9c2 cardiomyoblast cells grown on 24 well tissue culture plate, the DMEM-swelled MFSA-DEGDMA and MFSA-PEGDA hydrogel scaffolds were placed. The cells were allowed to proliferate for 48 h. After trypsinization, the cells were washed with PBS and incubated with Muse™ Count and Viability reagent at room temperature in dark. The analysis was completed within 5 min.
Cell cycle analysis. The proliferation capacity and health of the H9c2 cardiomyoblast cells upon contact with MFSA hydrogel scaffolds were assessed by the determination of DNA content using flow cytometry analysis (Muse™ Cell Analyzer). The cell culture was carried out as per the above procedure. Then the cell cycle analysis was done by using MUSE cell cycle kit as per the manufacturer's instructions. The kit utilizes a premixed reagent which includes the nuclear DNA intercalating stains propidium iodide (PI) which discriminates cells at different stages of the cell cycle (G0/G1, S and G2/M) based on the differential DNA content in each phase. After 48 h the scaffolds were removed and the cells were trypzinized and washed thrice with PBS by spinning at 4500 rpm. The washed cells were then fixed overnight using absolute ethanol at −20 °C. The fixed cells were then washed twice with PBS as above, incubated with cell cycle reagent at room temperature in dark for 30 min, and analysed on Muse flow-cytometer (Millipore, USA).
3.5. Statistical analysis
All experiments were carried out with of 5 or 6 samples from each group. The values are presented as means ± standard deviations. Statistical analysis was done with one way ANOVA using online calculator, Statistics Calculator version-3 beta and the level of significance was set at P < 0.05 for all calculations.4. Conclusions
Two novel bimodal hydrogels, MFSA-PEGDA and MFSA-DEGDMA were synthesized from hybrid biosynthetic comacromer MFSA by crosslinking with PEGDA and DEGDMA respectively for cardiac tissue engineering applications. These hydrogels were amphiphilic, contained appreciable water content, excellent pore morphology and mechanical properties comparable to native cardiac tissue. Moreover, both are biodegradable, hemocompatible and cytocompatible. The cardiomyoblast cells grown on contact with the hydrogels exhibited greater viability and normal cell cycle profile. The hydrogels supported long-term fibroblast cell viability, co-culture of cardiomyoblast cells and fibroblasts, promoted cell adhesion and crowding under hydrodynamic culture conditions, which are attributed to the inherent biological recognition characteristics. The cellular and extra cellular responses of the MFSA hydrogels signified their potent application for cardiac tissue engineering. MFSA-PEGDA hydrogel was found to possess slightly superior characteristics when compared with MFSA-DEGDMA.Acknowledgements
The authors acknowledge the Director, SCTIMST and Head, BMT Wing, SCTIMST, Thiruvananthapuram-695012 for providing the facilities to carry out this work and Department of Science & Technology, New Delhi, Government of India and KSCST&E, Kerala, India for financial aids.References
- J. F. Mano, G. A. Silva, H. S. Azevedo, P. B. Malafaya, R. A. Sousa, S. S. Silva, L. F. Boesel, J. M. Oliveira, T. C. Santos, A. P. Marques, N. M. Neves and R. L. Reis, J. R. Soc., Interface, 2007, 4, 999–1030 CrossRef CAS PubMed.
- J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS.
- A. M. Kloxin, M. W. Tibbitt and K. S. Anseth, Nat. Protoc., 2010, 5, 1867–1887 CrossRef CAS PubMed.
- J. Liu, D. Gao, H.-F. Li and J.-M. Lin, Lab Chip, 2009, 9, 1301–1305 RSC.
- C. Tu, Q. Cai, J. Yang, Y. Wan, J. Bei and S. Wang, Polym. Adv. Technol., 2003, 14, 565–573 CrossRef CAS PubMed.
- J. Gao, P. M. Crapo and Y. Wang, Tissue Eng., 2006, 12, 917–925 CrossRef CAS PubMed.
- F. P. W. Melchels, M. A. N. Domingos, T. J. Klein, J. Malda, P. J. Bartolo and D. W. Hutmacher, Prog. Polym. Sci., 2012, 37, 1079–1104 CrossRef PubMed.
- E. L. Baker, R. T. Bonnecaze and M. H. Zaman, Biophys. J., 2009, 97, 1013–1021 CrossRef CAS PubMed.
- H. Geckil, F. Xu, X. Zhang, S. Moon and U. Demirci, Nanomedicine, 2010, 5, 469–484 CrossRef CAS PubMed.
- S. Mendis, K. Thygesen, K. Kuulasmaa, S. Giampaoli, M. Mahonen, K. Ngu Blackett, L. Lisheng and Writing group on behalf of the participating experts of the WHO consultation for revision of WHO definition of myocardial infarction, Int. J. Epidemiol., 2011, 40, 139–146 CrossRef PubMed.
- V. L. Roger, A. S. Go, D. M. Lloyd-Jones, E. J. Benjamin, J. D. Berry, W. B. Borden, D. M. Bravata, S. Dai, E. S. Ford, C. S. Fox, H. J. Fullerton, C. Gillespie, S. M. Hailpern, J. A. Heit, V. J. Howard, B. M. Kissela, S. J. Kittner, D. T. Lackland, J. H. Lichtman, L. D. Lisabeth, D. M. Makuc, G. M. Marcus, A. Marelli, D. B. Matchar, C. S. Moy, D. Mozaffarian, M. E. Mussolino, G. Nichol, N. P. Paynter, E. Z. Soliman, P. D. Sorlie, N. Sotoodehnia, T. N. Turan, S. S. Virani, N. D. Wong, D. Woo, M. B. Turner and American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Circulation, 2012, 125, e2–e220 CrossRef PubMed.
- G. T. Finosh and M. Jayabalan, Biomaterials, 2012, 2, 1–14 CAS.
- C. V. C. Bouten, P. Y. W. Dankers, A. Driessen-Mol, S. Pedron, A. M. A. Brizard and F. P. T. Baaijens, Adv. Drug Delivery Rev., 2011, 63, 221–241 CrossRef CAS PubMed.
- M. Radisic, H. Park, S. Gerecht, C. Cannizzaro, R. Langer and G. Vunjak-Novakovic, Philos. Trans. R. Soc., B, 2007, 362, 1357–1368 CrossRef CAS PubMed.
- N. Annabi, K. Tsang, S. M. Mithieux, M. Nikkhah, A. Ameri, A. Khademhosseini and A. S. Weiss, Adv. Funct. Mater., 2013, 23, 4950–4959 CrossRef CAS PubMed.
- J. A. Rowley, G. Madlambayan and D. J. Mooney, Biomaterials, 1999, 20, 45–53 CrossRef CAS.
- D. M. Nelson, Z. Ma, K. L. Fujimoto, R. Hashizume and W. R. Wagner, Acta Biomater., 2011, 7, 1–15 CrossRef CAS PubMed.
- I. Djordjevic, N. R. Choudhury, N. K. Dutta and S. Kumar, Polym. Int., 2011, 60, 333–343 CrossRef CAS PubMed.
- J. Kim, T. E. Hefferan, M. J. Yaszemski and L. Lu, Tissue Eng., Part A, 2009, 15, 2299–2307 CrossRef CAS PubMed.
- A. D. Vilaeti, K. Dimos, E. S. Lampri, P. Mantzouratou, N. Tsitou, I. Mourouzis, D. L. Oikonomidis, A. Papalois, C. Pantos, V. Malamou-Mitsi, S. Agathopoulos and T. M. Kolettis, Int. J. Cardiol., 2013, 165, 278–284 CrossRef PubMed.
- V. Catto, S. Farè, G. Freddi and M. C. Tanzi, ISRN Vasc. Med., 2014, 2014, 1–27 CrossRef PubMed.
- Q.-Z. Chen, H. Ishii, G. A. Thouas, A. R. Lyon, J. S. Wright, J. J. Blaker, W. Chrzanowski, A. R. Boccaccini, N. N. Ali, J. C. Knowles and S. E. Harding, Biomaterials, 2010, 31, 3885–3893 CrossRef CAS PubMed.
- J. P. Bruggeman, B.-J. de Bruin, C. J. Bettinger and R. Langer, Biomaterials, 2008, 29, 4726–4735 CrossRef CAS PubMed.
- S.-L. Liang, W. D. Cook, G. A. Thouas and Q.-Z. Chen, Biomaterials, 2010, 31, 8516–8529 CrossRef CAS PubMed.
- D. B. Jennings, M. Ehrenshaft, D. M. Pharr and J. D. Williamson, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 15129–15133 CrossRef CAS.
- M. Dadsetan, M. Pumberger, M. E. Casper, K. Shogren, M. Giuliani, T. Ruesink, T. E. Hefferan, B. L. Currier and M. J. Yaszemski, Acta Biomater., 2011, 7, 2080–2090 CrossRef CAS PubMed.
- P. Thevenot, W. Hu and L. Tang, Curr. Top. Med. Chem., 2008, 8, 270–280 CrossRef CAS.
- B. G. Keselowsky, D. M. Collard and A. J. García, J. Biomed. Mater. Res., Part A, 2003, 66, 247–259 CrossRef PubMed.
- G. Vunjak-Novakovic, N. Tandon, A. Godier, R. Maidhof, A. Marsano, T. P. Martens and M. Radisic, Tissue Eng., Part B, 2010, 16, 169–187 CrossRef PubMed.
- M. Kharaziha, M. Nikkhah, S.-R. Shin, N. Annabi, N. Masoumi, A. K. Gaharwar, G. Camci-Unal and A. Khademhosseini, Biomaterials, 2013, 34, 6355–6366 CrossRef CAS PubMed.
- A. Simionescu, J. B. Schulte, G. Fercana and D. T. Simionescu, Int. J. Inflammation, 2011, 2011, 958247 Search PubMed.
- J. Sun and H. Tan, Materials, 2013, 6, 1285–1309 CrossRef CAS PubMed.
- H. Jin Lee and G. H. Kim, RSC Adv., 2012, 2, 7578 RSC.
- J. Wang, C. J. Bettinger, R. S. Langer and J. T. Borenstein, Organogenesis, 2010, 6, 212–216 CrossRef PubMed.
- M. C. Serrano, E. J. Chung and G. A. Ameer, Adv. Funct. Mater., 2010, 20, 192–208 CrossRef CAS PubMed.
- K. Y. Tserng and S. J. Jin, J. Biol. Chem., 1991, 266, 2924–2929 CAS.
- G. Sailakshmi, T. Mitra and A. Gnanamani, Prog. Biomater., 2013, 2, 1–12 CrossRef.
- K. K. Mäkinen and M. M. Hämäläinen, J. Nutr., 1985, 115, 890–899 Search PubMed.
- S. J. Angyal, in Glycoscience, ed. P. D. A. E. Stütz, Springer, Berlin Heidelberg, 2001, pp. 1–14 Search PubMed.
- M. Jayabalan, V. Thomas and P. K. Sreelatha, Biomed. Mater. Eng., 2000, 10, 57–71 CAS.
- P. M. Crapo, J. Gao and Y. Wang, J. Biomed. Mater. Res., Part A, 2008, 86, 354–363 CrossRef PubMed.
- J. M. Kemppainen and S. J. Hollister, J. Biomed. Mater. Res., Part A, 2010, 94, 9–18 CrossRef PubMed.
- M. Frydrych and B. Chen, J. Mater. Chem. B, 2013, 1, 6650–6661 RSC.
- K. Pal, A. K. Banthia and D. K. Majumdar, Des. Monomers Polym., 2009, 12, 197–220 CrossRef CAS PubMed.
- J. Wei, T. Igarashi, N. Okumori, T. Igarashi, T. Maetani, B. Liu and M. Yoshinari, Biomed. Mater., 2009, 4, 045002 CrossRef PubMed.
- X. Z. Shu, K. Ghosh, Y. Liu, F. S. Palumbo, Y. Luo, R. A. Clark and G. D. Prestwich, J. Biomed. Mater., Res. A, 2004, 68, 365–375 CrossRef PubMed.
- A. Vashist, A. Vashist, Y. K. Gupta and S. Ahmad, J. Mater. Chem. B, 2013, 2, 147–166 RSC.
- M. C. Cushing and K. S. Anseth, Science, 2007, 316, 1133–1134 CrossRef CAS PubMed.
- A. Patel, A. K. Gaharwar, G. Iviglia, H. Zhang, S. Mukundan, S. M. Mihaila, D. Demarchi and A. Khademhosseini, Biomaterials, 2013, 34, 3970–3983 CrossRef CAS PubMed.
- D. Kafouris, F. Kossivas, C. Constantinides, N. Q. Nguyen, C. Wesdemiotis and C. S. Patrickios, Macromolecules, 2013, 46, 622–630 CrossRef CAS.
- V. Karageorgiou and D. Kaplan, Biomaterials, 2005, 26, 5474–5491 CrossRef CAS PubMed.
- Q. L. Loh and C. Choong, Tissue Eng., Part B, 2013, 19, 485–502 CrossRef CAS PubMed.
- L. R. Madden, D. J. Mortisen, E. M. Sussman, S. K. Dupras, J. A. Fugate, J. L. Cuy, K. D. Hauch, M. A. Laflamme, C. E. Murry and B. D. Ratner, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(34), 15211–15216 CrossRef CAS PubMed.
- J. Rnjak-Kovacina, S. G. Wise, Z. Li, P. K. M. Maitz, C. J. Young, Y. Wang and A. S. Weiss, Biomaterials, 2011, 32, 6729–6736 CrossRef CAS PubMed.
- Y.-Q. Xiang, Y. Zhang and D.-J. Chen, Polym. Int., 2006, 55, 1407–1412 CrossRef CAS PubMed.
- F. Gnanaprakasam Thankam, J. Muthu, V. Sankar and R. Kozhiparambil Gopal, Colloids Surf., B, 2013, 107, 137–145 CrossRef CAS PubMed.
- M. Tanaka, T. Motomura, N. Ishii, K. Shimura, M. Onishi, A. Mochizuki and T. Hatakeyama, Polym. Int., 2000, 49, 1709–1713 CrossRef CAS.
- B. Bhana, R. K. Iyer, W. L. K. Chen, R. Zhao, K. L. Sider, M. Likhitpanichkul, C. A. Simmons and M. Radisic, Biotechnol. Bioeng., 2010, 105, 1148–1160 CAS.
- W.-H. Zimmermann, I. Melnychenko and T. Eschenhagen, Biomaterials, 2004, 25, 1639–1647 CrossRef CAS.
- M. Isaka, T. Nishibe, Y. Okuda, M. Saito, T. Seno, K. Yamashita, Y. Izumisawa, T. Kotani and K. Yasuda, Ann. Thorac. Cardiovasc. Surg. Off. J. Assoc. Thorac. Cardiovasc. Surg. Asia, 2006, 12, 37–41 Search PubMed.
- S. Pok, J. D. Myers, S. V. Madihally and J. G. Jacot, Acta Biomater., 2013, 9, 5630–5642 CrossRef CAS PubMed.
- D. G. Barrett and M. N. Yousaf, Molecules, 2009, 14, 4022–4050 CrossRef CAS PubMed.
- B. Balakrishnan and A. Jayakrishnan, Biomaterials, 2005, 26, 3941–3951 CrossRef CAS PubMed.
- Z. Peng and Y. Shen, Polym.-Plast. Technol. Eng., 2011, 50, 245–250 CrossRef CAS.
- O. K. Baskurt and H. J. Meiselman, Indian J. Exp. Biol., 2007, 45, 25–31 CAS.
- Y. B. J. Aldenhoff and L. H. Koole, Eur. Cells Mater., 2003, 5, 61–67 CAS.
- E.-E. Hago and X. Li, Adv. Mater. Sci. Eng., 2013, 2013, 1–8 CrossRef PubMed.
- J. Fukuda, A. Khademhosseini, Y. Yeo, X. Yang, J. Yeh, G. Eng, J. Blumling, C.-F. Wang, D. S. Kohane and R. Langer, Biomaterials, 2006, 27, 5259–5267 CrossRef CAS PubMed.
- L. Klouda, M. C. Hacker, J. D. Kretlow and A. G. Mikos, Biomaterials, 2009, 30, 4558–4566 CrossRef CAS PubMed.
- D. Ribble, N. B. Goldstein, D. A. Norris and Y. G. Shellman, BMC Biotechnol., 2005, 5, 12 CrossRef PubMed.
- Y.-N. Kim, K. H. Koo, J. Y. Sung, U.-J. Yun and H. Kim, Int. J. Cell Biol., 2012, 2012, 306879 Search PubMed.
- C. Z. Chen and M. Raghunath, Fibrog. Tissue Repair, 2009, 2, 7 CrossRef PubMed.
- H. Ju, S. Zhao, D. S. Jassal and I. M. Dixon, Cardiovasc. Res., 1997, 35, 223–232 CrossRef CAS.
- C. Frantz, K. M. Stewart and V. M. Weaver, J. Cell Sci., 2010, 123, 4195–4200 CrossRef CAS PubMed.
- B. Wang, M. E. Tedder, C. E. Perez, G. Wang, A. L. de Jongh Curry, F. To, S. H. Elder, L. N. Williams, D. T. Simionescu and J. Liao, J. Mater. Sci.: Mater. Med., 2012, 23, 1835–1847 CrossRef CAS PubMed.
- N. Sultana and T. H. Khan, J. Nanomater., 2012, 2012, 1–8 Search PubMed.
- R. Vasita and D. S. Katti, Int. J. Nanomedicine, 2012, 7, 61–71 CAS.
- N. Annabi, J. W. Nichol, X. Zhong, C. Ji, S. Koshy, A. Khademhosseini and F. Dehghani, Tissue Eng., Part B, 2010, 16, 371–383 CrossRef CAS PubMed.
- G. Y. Huang, L. H. Zhou, Q. C. Zhang, Y. M. Chen, W. Sun, F. Xu and T. J. Lu, Biofabrication, 2011, 3, 012001 CrossRef PubMed.
- C. Fan, L. Liao, C. Zhang and L. Liu, J. Mater. Chem. B, 2013, 1, 4251–4258 RSC.
- J. Tan, R. A. Gemeinhart, M. Ma and W. M. Saltzman, Biomaterials, 2005, 26, 3663–3671 CrossRef CAS PubMed.
- S. S. Ng, C. Li and V. Chan, Interface Focus, 2011, 1, 777–791 CrossRef PubMed.
- L. Bacáková, E. Filová, F. Rypácek, V. Svorcík and V. Starý, Physiol. Bohemoslov., 2004, 53(suppl 1), S35–S45 Search PubMed.
- F. X. Li, J. W. Zhu, C. J. Hogan and J. DeGregori, Mol. Cell. Biol., 2003, 23, 3607–3622 CrossRef CAS.
- C. Jayat and M.-H. Ratinaud, Biol. Cell, 1993, 78, 15–25 CrossRef CAS.
- F. Boccafoschi, N. Rajan, J. Habermehl and D. Mantovani, Macromol. Biosci., 2007, 7, 719–726 CrossRef CAS PubMed.
- E. Y. Kim, J. B. Lee, H. Y. Park, C. J. Jeong, K. Z. Riu and S. P. Park, J. Reprod. Dev., 2011, 57, 346–354 CrossRef CAS PubMed.
- R. Lu, F. Bian, J. Lin, Z. Su, Y. Qu, S. C. Pflugfelder and D.-Q. Li, PLoS One, 2012, 7, e38825 CAS.
- A. Hussain, G. Collins, D. Yip and C. H. Cho, Biotechnol. Bioeng., 2013, 110, 637–647 CrossRef CAS PubMed.
- Q. Luo, G. Song, Y. Song, B. Xu, J. Qin and Y. Shi, Cytotechnology, 2009, 61, 1–10 CrossRef PubMed.
- T. C. McDevitt and S. P. Palecek, Curr. Opin. Biotechnol., 2008, 19, 527–533 CrossRef CAS PubMed.
- R. E. Akins, N. A. Schroedl, S. R. Gonda and C. R. Hartzell, In Vitro Cell. Dev. Biol.: Anim., 1997, 33, 337–343 CrossRef CAS PubMed.
- A. Teo, A. Mantalaris and M. Lim, J. Regener. Med. Tissue Eng., 2012, 1, 4 CrossRef PubMed.
- M. T. Lam and J. C. Wu, Expert Rev. Cardiovasc. Ther., 2012, 10, 1039–1049 CrossRef CAS PubMed.
- M. Arnal-Pastor, J. C. Chachques, M. Monlen and A. Valls-Lluch, in Regenerative Medicine and Tissue Engineering, ed. J. A. Andrades, InTech, 2013 Search PubMed.
- G. Perri, A. Polito, C. Esposito, S. B. Albanese, P. Francalanci, G. Pongiglione and A. Carotti, Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg., 2012, 41, 1320–1325 CrossRef PubMed.
- F. G. Thankam and J. Muthu, J. Biomed. Mater. Res., Part A, 2014, 102(7), 2238–2247 CrossRef PubMed.
- M. Vandrovcová, T. Douglas, D. Hauk, B. Grössner-Schreiber, J. Wiltfang, L. Bačáková and P. H. Warnke, Physiol. Bohemoslov., 2011, 60, 797–813 Search PubMed.
- F. Gnanaprakasam Thankam and J. Muthu, RSC Adv., 2013, 3, 24509 RSC.
- H. Yokoyama, T. Danjo, K. Ogawa and H. Wakabayashi, J. Fish Dis., 1997, 20, 281–286 Search PubMed.
- G. Condorelli, U. Borello, L. D. Angelis, M. Latronico, D. Sirabella, M. Coletta, R. Galli, G. Balconi, A. Follenzi, G. Frati, M. G. C. D. Angelis, L. Gioglio, S. Amuchastegui, L. Adorini, L. Naldini, A. Vescovi, E. Dejana and G. Cossu, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 10733–10738 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04448k |
| This journal is © The Royal Society of Chemistry 2015 |