Preparation of single-handed helical carbonaceous nanotubes using 3-aminophenol-formaldehyde resin†
Hao Chen,
Yi Li,
Xianhui Tang,
Baozong Li,
Chuanyong Zhang and
Yonggang Yang*
Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Department of Polymer Science and Engineering, College of Chemistry, Chemical Engineering and Material Science, Soochow University, Suzhou 215123, P. R. China. E-mail: ygyang@suda.edu.cn; Fax: +86 512 6588 2052; Tel: +86 512 65880047
First published on 9th April 2015
Abstract
Single-handed helical 3-aminophenol-formaldehyde resin nanotubes were prepared using an external supramolecular templating method; the nanotubes were formed via the adsorption and polycondensation of 3-aminophenol-formaldehyde resin oligomers on the surface of organic self-assemblies. After carbonization at 1400 °C, single-handed helical carbonaceous nanotubes were obtained. A Raman spectrum, X-ray diffraction pattern, and high resolution transmission electron microscopy image showed that the carbon in the nanotubes was partially crystallized. Circular dichroism (CD) spectra indicated that the carbonaceous nanotubes exhibited optical activity; it was proposed that this optical activity originated from the stacking of aromatic rings. The CD spectrum was simulated using time-dependent density functional theory. When poly(n-decyl-2-methylpropylsilane) was adsorbed on the surface of the helical carbonaceous nanotubes, it tended to stack in a one-handed fashion.
Introduction
With the development of nano- and micro-scale functionalized materials, such materials with helical morphologies have attracted much attention for their potential applications in asymmetric catalysis, enantioseparation, chirality detection, electromagnetic wave adsorption, and chiral optics.1–14 A variety of synthetic approaches have been developed for the preparation of materials with a helical morphology. For example, carbon microcoils have been prepared using a chemical vapor deposition (CVD) method, and a metal-catalyzed pyrolysis process.1–3 Silicon carbide nanosprings were prepared using a plasma enhanced chemical vapor deposition process.4 Silicon microtubes have been prepared via the evaporation of sodium from NaSi disks.5 ZnO nanosprings were prepared using a solid-vapor approach.6 Although materials with helical morphologies can be obtained, it is difficult to obtain single-handed materials using these approaches. With the development of the chiral supramolecular templating approach, SiO2, Ta2O5, TiO2, CdS, ZrO2, polybissilsesquioxane, and polypyrrole have been successfully prepared with single-handed helical morphologies.15–29 The optical chirality of these materials has been studied using circular dichroism (CD), and diffuse reflectance circular dichroism (DRCD).Although several methods have been developed for the separation of chiral single-walled carbon nanotubes, these processes are typically laborious and time-intensive. Recently, two methods have been developed for the production of single-handed carbonaceous nanotubes: one method involves the pyrolysis of single-handed helical polypyrrole nanotubes,27 and the other involves the calcination and removal of silica from single-handed helical aromatic ring-bridged polybissilsesquioxane nanotubes.28,29 Herein, we show that single-handed helical carbonaceous nanotubes can be prepared via the pyrolysis of single-handed helical 3-aminophenol-formaldehyde resin nanotubes. Circular dichroism spectra indicated that the carbonaceous nanotubes exhibited optical activity; it was proposed that this optical activity originated from the stacking of aromatic rings. As far as we know, this is the first report of the preparation of single-handed helical carbonaceous nanotubes with optical activity via the carbonization of 3-aminophenol-formaldehyde resin.
Experimental section
General methods
Field-emission scanning electron microscopy (FESEM) images were taken using a Hitachi S-4800 instrument operated at 3.0 kV. Transmission electron microscopy (TEM) images were obtained using a TecnaiG220 instrument operated at 200 kV. High-resolution TEM (HR-TEM) images were obtained using a FEI Tecnai G2 F20S-TWIN instrument. Wide angle X-ray diffraction (WAXRD) patterns were obtained using an X’Pert-Pro MPD X-ray diffractometer, using Cu Kα radiation with a Ni filter (1.542 Å). Ultraviolet-visible (UV-vis) and CD spectra were recorded on an AVIV 410 spectrophotometer with a 1.0 nm bandwidth at 25 °C, using single scans at an average time of 0.1 s, using a 1.0 cm cell. Elemental analysis was performed on a Perkin Elmer series II CHNS/O analyzer 2400. Raman spectra were obtained using a Jobin Yvon Horiba HR 800 Labram confocal microprobe Raman system. The sizes of the slit and pinhole were 100 and 400 μm, respectively. The Ar laser excitation wavelength was 514.5 nm, and the highest laser power was 10 mW. DRCD and DRUV-vis spectra were recorded using a JASCO J-815 spectropolarimeter fitted with DRCD apparatus with a 10.0 nm bandwidth, 1.0 nm data pitch, and 100 nm min−1 scan speed.Results and discussion
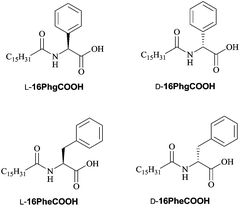
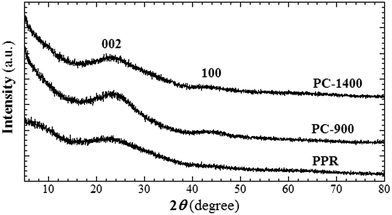
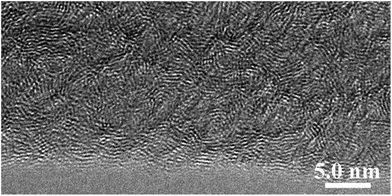
Chiral low-molecular-weight amphiphiles (LMWAs) can self-assemble into various helical nanostructures; these self-assemblies typically serve as templates to control the handedness of one-dimensional materials in sol–gel transcription methods. In this work, a pair of chiral LMWAs, L- and D-16PhgCOOH (Fig. 1), were synthesized via the acylation of phenylglycine with palmitoyl chloride, according to a literature method.30 These chiral LMWAs were used to prepare single-handed helical 3-aminophenol-formaldehyde resin nanotubes. FESEM and TEM images of the helical nanotubes are shown in Fig. 2a and b and 3a and b. Left-handed (MPR) and right-handed (PPR) helical nanotubes were obtained using L- and D-16PhgCOOH, respectively. The lengths of the nanotubes were of the order of several microns. The outer diameters, inner diameters, and helical pitches of these nanotubes were 60–100, 20–30, and 250–300 nm, respectively, and their termini exhibited a rectangular-like structure (Fig. 2a and b). After the 3-aminophenol-formaldehyde resin nanotubes were pyrolyzed at 900 °C for 4.0 h, single-handed helical N-doped carbonaceous nanotubes MC-900 and PC-900 were obtained from MPR and PPR, respectively (Fig. 2c and d and 3c and d). For PC-900, the elemental analysis results indicated that the carbon and nitrogen contents were 83.61% and 4.36%, respectively. Nitrogen doping can enhance the surface polarity, electrical conductivity, and electron-donor tendencies of carbonaceous materials, enabling their application in CO2 capture, fuel cells, and catalysis.31 The outer diameters, inner diameters, and helical pitches of these nanotubes were 50–80, 10–25, and 250–300 nm. The carbonization process did not destroy the handedness of the helical nanotubes. However, the outer and inner diameters decreased because of thermal shrinkage. When the pyrolysis temperature was increased to 1400 °C, carbonaceous nanotubes MC-1400 and PC-1400 were obtained from MPR and PPR, respectively (Fig. 2e and f and 3e and f). The helical morphologies of the nanotubes remained the same. However, the inner diameters decreased to approximately 10 nm. For PC-1400, the elemental analysis results indicated that the carbon and nitrogen contents were 95.30% and 0.39%, respectively. Most of the nitrogen atoms were removed. A variety of highly ordered mesoporous carbonaceous materials have been synthesized via the pyrolysis of mesoporous phenolic resins.32,33 Herein, the single-handed helical carbonaceous nanotubes were obtained via the pyrolysis of 3-aminophenol-formaldehyde resin nanotubes, indicating that a wide variety of carbonaceous materials with chiral morphologies and tunable pore architectures could potentially be prepared using this approach.The Raman spectra for PC-900 and PC-1400 (measured using 514.5 nm excitation) are shown in Fig. 4. These two spectra were very similar. The G and D bands were observed at 1600 and 1358 cm−1, respectively. The IG/ID ratios for PC-900 and PC-1400 were approximately 0.36 and 0.65, respectively, indicating that the crystalline sizes in the two samples were of the order of several nanometers, and that they increased with increasing carbonization temperature.3 The WAXRD patterns for the right-handed helical 3-aminophenol-formaldehyde resin nanotubes and the carbonaceous nanotubes are shown in Fig. 5. The PPR nanotubes showed a broad peak at approximately 23.6°, indicating a lack of long-range ordered structure. For PC-900 and PC-1400, two broad peaks were identified at 2θ values of 22.6° and 43.3°, respectively, corresponding to the (002) and (100) planes for graphite; these results indicated that no large crystalline domains were present in the samples. The partially crystallized structure was clearly identified in the high-resolution TEM (HR-TEM) image (Fig. 6). The nanocrystals, which were constructed from several layers of graphene, were randomly arranged. The distance between neighboring layers was approximately 3.4 Å.
To understand the formation of the single-handed, helical, 3-aminophenol-formaldehyde resin nanotubes, FESEM images of the reaction mixtures were taken after different reaction times (Fig. 7). The mixture of L-16PhgCOOH and 3-aminophenol dissolved in anhydrous methanol formed a clear solution. After the addition of water, the reaction mixture became cloudy. Ten minutes later, nanofibers were observed in the FESEM image, and some of the nanofibers exhibited a right-handed morphology (Fig. 7a), which demonstrated that the organic self-assemblies had formed in the methanol/water mixture. One minute after the addition of formaldehyde, white precipitate appeared in the reaction mixture. Right-handed helical nanotubes were identified, as shown in the image in Fig. 7b; this indicated that the polycondensation reaction of 3-aminophenol with formaldehyde was very rapid, and thus the surface of the nanotubes solidified in a very short time. The subsequent stages of the reaction involved only further cross-linking of the resins.
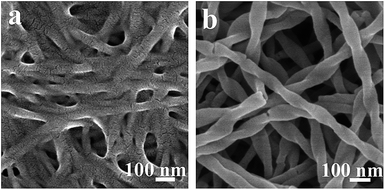 | ||
| Fig. 7 FESEM images of the reaction mixture taken after different reaction times. (a) 10 min after the addition of water, and (b) 1.0 min after the addition of formaldehyde. | ||
A possible formation mechanism can be described as follows (Scheme 1): first, the L-16PhgCOOH combined with the 3-aminophenol in the methanol solution through cationic–anionic interactions. When water was added to the mixture, the amphiphilic molecules self-assembled through non-covalent interactions to form helical fibrous aggregates. 3-Aminophenol was adsorbed on the outer surface of these nanofibers. Second, when formaldehyde was added to the reaction mixture, it reacted with 3-aminophenol to form 3-aminophenol-formaldehyde resin oligomers on the surface of the organic self-assemblies. Finally, after thermosetting and removal of the low-molecular-weight compounds, single-handed helical 3-aminophenol-formaldehyde resin nanotubes were obtained. The resins preserved the helical morphology of the organic self-assemblies.
CD and UV-vis spectra were measured for the carbonaceous nanotubes in methanol, using a nanotube concentration of 0.09 mg mL−1 and a 1.0 cm cell (Fig. 8a). Broad UV-vis absorbance bands were identified in the range of 240 to 700 nm. MC-900 and PC-900 exhibited opposing CD signals. The λθ=0 values appeared at about 540 nm. Previous results indicated that bisigned CD couplet signals can originate from the chiral stacking of aromatic rings.34–36 The CD spectra were similar to those of single-handed helical nanotubes containing twisted carbonaceous nanoribbons.37 Based on time-dependent density functional theory (TD-DFT) calculations, it was suggested that the optical activity originated from the stacking of aromatic rings. MC-1400 and PC-1400 also exhibited opposing CD signals. For MC-1400, two broad, positive CD signals were identified at 499 and 269 nm. The CD signals shown here might also have originated from such stacking.
 | ||
| Fig. 8 (a) CD and UV spectra for the single-handed helical carbonaceous nanotubes, and (b) TD-DFT simulated CD spectrum. | ||
To achieve a better understanding of the origin of the CD signals, the coronene dimer was used to model the real system in simulations of the CD spectra (Fig. 8b). As shown in Fig. S1,† the distance between the two coronene planes was fixed at approximately 3.40 Å, and the dihedral angle between the two coronene planes was approximately 13°. The UV and CD spectra were determined using TD-DFT, at the B3LYP/6-31G+(d,p) level of the theory, using the Gaussian quantum package, version G09.38 The calculated CD spectra based on the 50 lowest-energy singlet excited states were prepared by fitting each absorption wavelength to a Gaussian curve with a half-width at half height of 2685.83 cm−1. The highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) are shown in Fig. S2,† where the different colours on the orbitals indicate the different phases of the wavefunction. Considering the CD spectrum (Fig. 8b), there was a large positive signal at approximately 390 nm, which was composed of two important electronic excitations. One was the transition at 390.24 nm, which originated mainly from the HOMO − 1 to LUMO and HOMO to LUMO + 1 transitions with a rotatory strength of 31.88 × 10−40 cgs. The other was the transition at 388.14 nm, which was mainly caused by electron transfer from HOMO − 1 to LUMO + 1 with a rotatory strength of 18.91 × 10−40 cgs. In addition, there was another large positive signal around 294 nm, which mostly arises from the electron transfer from HOMO − 4 to LUMO + 1 and HOMO − 5 to LUMO with a rotatory strength of 389.69 × 10−40 cgs. As shown in Fig. S2,† the electron clearly transferred between the aromatic rings with the electronic excitation. During the carbonization process, varieties of aromatic compounds would be formed. The size of the aromatic rings within the carbonaceous walls was thought to be increased with increasing carbonization temperature. Although the experimental signals and the calculated CD signals based on the coronene dimer are not exactly same, the origin of the optical activity can be partially illustrated. For example, the calculated CD signal is at 294 nm and the experimental one is at 269 nm. The signal at 269 nm might be mainly attributed to smaller aromatic rings.37
To study the relationship between the helical pitch and the CD signals, straight carbonaceous nanotubes were prepared using phenylalanine derivatives (Fig. 1), and were carbonized at 1400 °C. FESEM and TEM images of the nanotubes are shown in Fig. S3.† The outer and inner diameters of these nanotubes were 50–100 and 20–30 nm, respectively. The CD signals were similar to those of MC-1400 and PC-1400 (Fig. 9a). For LC-Phe-1400, one broad positive CD signal was identified at 284 nm. Since the position of the CD signal is not related to the helical pitch length, it is likely that the CD signal also originated from the electron transfer between neighboring aromatic rings. However, the intensity of the CD signal increased with decreasing helical pitch length. It seems that more chiral stacking structures were formed in tightly twisted nanotubes. There was therefore no obvious relationship between the helical pitch and the CD signals.
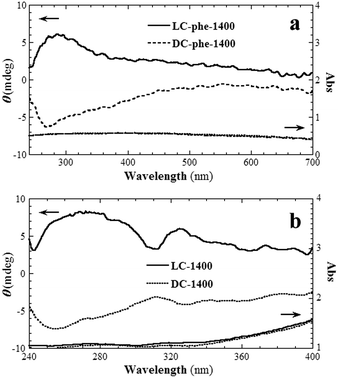 | ||
| Fig. 9 CD and UV spectra for (a) the straight carbonaceous nanotubes and (b) the single-handed helical carbonaceous nanotubes with adsorbed poly(n-decyl-2-methylpropyl)silane. | ||
The 3-aminophenol-formaldehyde resin nanotubes were obtained using a supramolecular templating approach; that is, they were formed via the adsorption and polycondensation of resin oligomers on the outer surface of organic self-assemblies. The chirality of the organic self-assemblies was transferred to the inner surface of the resin nanotubes (Fig. 5). The diffuse reflectance CD (DRCD) spectra of the nanotubes indicated that the aromatic rings at least partially stacked in a single-handed fashion (Fig. S4†); the obtained carbonaceous nanotubes therefore exhibited chirality on their inner surfaces. Poly(n-decyl-2-methylpropyl)silane is a kind of semi-rigid polymer. The chiral stacking of the polymer main chain can be controlled via the addition of chiral low-molecular-weight compounds.39 Because polydecylisobutylsilane is UV-active, it can be used as a chirality detector.40 Herein, it was found that the surfaces of MC-1400 and PC-1400 could drive poly(n-decyl-2-methylpropyl)silane (Mw = 5.31 × 104; PDI = 1.12) chains to stack in a right- and left-handed fashion, respectively (Fig. 9b). Bisigned CD couplet signals were identified at 320 nm, which originated from the electron transfer among the polysilane chains. These results indicate that the surface of the carbonaceous nanotubes was chiral.
Conclusions
Single-handed helical carbonaceous nanotubes with optical activity were obtained by the carbonization of 3-aminophenol-formaldehyde resin nanotubes at 1400 °C. Nitrogen-doped carbonaceous nanotubes were obtained at a lower carbonization temperature. The CD spectra and TD-DFT calculations suggested that the optical activity originated from the stacking of aromatic rings. The chirality on the surface of the carbonaceous nanotubes was successfully transferred to the stacking of polysilane chains, indicating that the carbonaceous nanotubes have the potential to be applied for enantioseparation and asymmetric catalysis. The results shown here not only give us a better understanding of the optical activity of the carbonaceous nanotubes, but can also help us to prepare carbonaceous materials with a variety of morphologies.Acknowledgements
This work was supported by the Priority Academic Program Development of Jiangsu High Education Institutions (PAPD), and the National Natural Science Foundation of China (nos 51473106 and 21274098). The authors thank Prof. Mingliang Wang for performing the quantum chemistry calculations.Notes and references
- S. Motojima, S. Hoshiya and Y. Hishikawa, Carbon, 2003, 41, 2658 CrossRef CAS.
- Y. Qin, Z. Zhang and Z. Cui, Carbon, 2003, 41, 3072 CrossRef CAS.
- X. Chen, S. Yang, S. Motojima and M. Ichihara, Mater. Lett., 2005, 59, 854 CrossRef CAS PubMed.
- D. Zhang, A. Alkhateeb, H. Han, H. Mahmood, D. N. McIlroy and M. G. Norton, Nano Lett., 2003, 3, 983 CrossRef CAS.
- H. Morito and H. Yamane, Angew. Chem., Int. Ed., 2010, 49, 3638 CrossRef CAS PubMed.
- X. Y. Kong and Z. L. Wang, Nano Lett., 2003, 3, 1625 CrossRef CAS.
- X. Y. Gao, Y. Ding, W. Mai, W. L. Hughes, C. Lao and Z. L. Wang, Science, 2005, 309, 1700 CrossRef PubMed.
- S. Che, Z. Liu, T. Ohsuna, K. Sakamoto, O. Terasaki and T. Tatsumi, Nature, 2004, 429, 281 CrossRef CAS PubMed.
- T. Ohsuna, Z. Liu, S. Che and O. Terasaki, Small, 2005, 2, 233 CrossRef PubMed.
- J. Xie, H. Qiu and S. Che, Chem.–Eur. J., 2012, 18, 2559 CrossRef CAS PubMed.
- H. Jin, H. Qiu, Y. Sakamoto, P. Shu, O. Terasak and S. Che, Chem.–Eur. J., 2008, 14, 6413 CrossRef CAS PubMed.
- B. Cao, H. Xu and C. Mao, Angew. Chem., Int. Ed., 2011, 50, 6264 CrossRef CAS PubMed.
- C. Mao, F. Wang and B. Cao, Angew. Chem., Int. Ed., 2012, 51, 6411 CrossRef CAS PubMed.
- F. Wang, S. L. Nimmo, B. Cao and C. Mao, Chem. Sci., 2012, 3, 2639 RSC.
- J. H. Jung, Y. Ono, K. Hanabusa and S. Shinkai, J. Am. Chem. Soc., 2000, 122, 5008 CrossRef CAS.
- K. J. C. van Bommel, A. Friggeri and S. Shinkai, Angew. Chem., Int. Ed., 2003, 42, 980 CrossRef CAS PubMed.
- T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401 CrossRef CAS PubMed.
- X. Wu, S. Ji, Y. Li, B. Li, X. Zhu, K. Hanabusa and Y. Yang, J. Am. Chem. Soc., 2009, 131, 5986 CrossRef CAS PubMed.
- Y. Yang, M. Suzuki, S. Owa, H. Shirai and K. Hanabusa, J. Am. Chem. Soc., 2007, 129, 581 CrossRef CAS PubMed.
- J. J. E. Moreau, L. Vellutini, M. Wong Chi Man and C. Bied, J. Am. Chem. Soc., 2001, 123, 1509 CrossRef CAS.
- S. Kobayashi, N. Hamasaki, M. Suzuki, M. Kimura, H. Shirai and K. Hanabusa, J. Am. Chem. Soc., 2002, 124, 6550 CrossRef CAS PubMed.
- S. Liu, L. Han, Y. Duan, S. Asahina, O. Terasaki, Y. Cao, B. Liu, L. Ma, J. Zhang and S. Che, Nat. Commun., 2012, 3, 2215 Search PubMed.
- C. Zhang, H. Huo, Y. Li, B. Li and Y. Yang, Mater. Lett., 2013, 102–103, 50 CrossRef CAS PubMed.
- C. Zhang, S. Wang, H. Huo, Z. Huang, Y. Li, B. Li and Y. Yang, Chem.–Asian J., 2013, 8, 709 CrossRef CAS PubMed.
- C. Zhang, S. Wang, H. Huo, Y. Li, B. Li and Y. Yang, Mater. Lett., 2012, 88, 23 CrossRef CAS PubMed.
- H. Huo, S. Wang, S. Lin, Y. Li, B. Li and Y. Yang, J. Mater. Chem. A, 2014, 2, 333 CAS.
- S. Liu, Y. Duan, X. Feng, J. Yang and S. Che, Angew. Chem., Int. Ed., 2013, 52, 6858 CrossRef CAS PubMed.
- C. Zhang, Y. Li, B. Li and Y. Yang, Chem.–Asian J., 2013, 8, 2714 CrossRef CAS PubMed.
- S. Lin, Y. Fu, Y. Sang, Y. Li, B. Li and Y. Yang, AIMS Mater. Sci., 2014, 1, 1 CrossRef.
- M. Gerova, F. Rodrigues, J.-F. Lamère, J.-F. Dobrev and S. Fery-Forgues, J. Colloid Interface Sci., 2008, 319, 526 CrossRef CAS PubMed.
- J. Wei, D. Zhou, Z. Sun, Y. Deng, Y. Xia and D. Zhao, Adv. Funct. Mater., 2013, 23, 2322 CrossRef CAS PubMed.
- Y. Meng, D. Gu, F. Zhang, Y. Shi, L. Cheng, D. Feng, Z. Wu, Z. Chen, Y. Wan, A. Stein and D. Zhao, Chem. Mater., 2006, 18, 4447 CrossRef CAS.
- F. Zhang, Y. Meng, D. Gu, Y. Yan, C. Yu, B. Tu and D. Zhao, J. Am. Chem. Soc., 2005, 127, 13508 CrossRef CAS PubMed.
- A. D’Urso, R. Randazzo, L. L. Faro and R. Purrello, Angew. Chem., Int. Ed., 2010, 49, 108 CrossRef PubMed.
- M. Wolffs, S. J. George, Ž. Tomović, S. C. J. Merkers, A. P. H. J. Schenning and E. W. Meijier, Angew. Chem., Int. Ed., 2007, 46, 8203 CrossRef CAS PubMed.
- A. Tsuda, M. A. Alam, T. Harada, T. Yamaguchi, N. Ishiiand and T. Aida, Angew. Chem., Int. Ed., 2007, 46, 8198 CrossRef CAS PubMed.
- H. Huo, Y. Li, Y. Yuan, S. Lin, B. Li, M. Wang and Y. Yang, Chem.–Asian J., 2014, 9, 2866 CrossRef CAS PubMed.
- M. J. Frisch, et al., Gaussian 09, Revision A.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- H. Nakashima, J. R. Koe, K. Torimitsu and M. Fujiki, J. Am. Chem. Soc., 2001, 123, 4847 CrossRef CAS.
- Y. Nakano, F. Ichiyanagi, M. Naito, Y. Yang and M. Fujiki, Chem. Commun., 2012, 48, 6636 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: FESEM and TEM images and DRCD spectra of carbonaceous samples and calculated figures of TD-DFT calculations. See DOI: 10.1039/c5ra04437e |
| This journal is © The Royal Society of Chemistry 2015 |