Synthesis and characterization of acrylic polyols and polymers from soybean oils for pressure-sensitive adhesives†
Yonghui Li and
Xiuzhi Susan Sun*
Bio-Materials and Technology Lab, Department of Grain Science and Industry, Kansas State University, Manhattan, KS 66506, USA. E-mail: xss@ksu.edu; Fax: +1 785-532-7193; Tel: +1 785-532-4077
First published on 8th May 2015
Abstract
A new class of acrylic polyols was synthesized from epoxidized soybean oil (ESO) and free-radically polymerized via UV irradiation to form pressure-sensitive adhesives (PSAs). ESO first was partially acrylated, then the remaining epoxy groups were dihydroxylated to make acrylic polyols. The acrylic polyols were characterized with Fourier transform infrared spectroscopy, 1H nuclear magnetic resonance, and hydroxyl value measurements. The degree of acrylation and hydroxyl functionality were carefully controlled to obtain polymers with a good balance of flexibility, crosslinking, and polarity, which are key attributes of PSAs. The glass transition temperature, rubbery plateau modulus, and cross-link density of polymers increased as the amount of acrylic polyol and the acrylate functionality of the resin increased. Biobased PSA with a good balance of peel strength (>4 N in−1), tack (>7 N in−1), and shear resistance (>50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 min) was achieved. Positive correlations between the mechanical performance and viscoelastic responses of a frequency sweep of the PSAs were found.
000 min) was achieved. Positive correlations between the mechanical performance and viscoelastic responses of a frequency sweep of the PSAs were found.
1. Introduction
Pressure-sensitive adhesives (PSAs) adhere to a variety of substrates when applied with light pressure.1 The primary bonding mechanism of PSAs is a polar attraction to the substrate surface (e.g., van der Waals interaction, electrostatic forces, and hydrogen bonding).2 PSAs have been widely used for tapes, labels, graphics, medical and many other applications, and demand is increasing.3 Many PSAs are made from petroleum-based acrylate monomers, such as 2-ethylhexyl acrylate (2-EHA), butyl acrylate, and isooctyl acrylate.3,4 Developing acrylates from renewable resources to partially or fully replace their petrochemical counterparts in PSAs would reduce the dependency on limited petroleum resources.World plant oil production has increased from 90 million metric tons in 2000 to nearly 170 million metric tons in 2013, and production is increasing. Plant oil is one of the most attractive renewable chemicals for potentially adhesive applications.5 Plant oils are mixtures of triglycerides with varying compositions of saturated and unsaturated fatty acids. Fatty acid distribution may differ depending on the crop, season, and growing conditions.6 Various oil derivatives have been synthesized via oleochemistry and applied in resins, composites, coatings, adhesives, surfactants, lubricants, cosmetic products, as well as biomedical uses,7–14 but only a few studies have been carried out to develop PSAs from plant oils.15–20
Plant oil-derived acrylates have been available in the past few years,21–23 and several papers have documented polymerization of acrylated epoxidized soybean oil (AESO)24,25 and acrylated methyl oleate (AMO)15,26,27 for PSAs. However, the adhesives were too weak in tack strength and peel adhesion (AESO-based) or in shear resistance (AMO-based) for practical applications. AESO usually had 2–4 acrylate groups per triglyceride, meaning polymerized AESO products were highly cross-linked and did not possess enough softness and flexibility to wet the substrate adequately to form a good bond25 despite their good cohesive strength and shear resistance. Polymerized AMO was a linear polymer with relatively good peel adhesion and initial tack, but its shear resistance was only 10 min.26 Copolymerization of AMO with a multi-functional acrylate increased the polymer shear resistance to about 6000 min, but the peel strength was reduced to less than 1 N in−1.15 In addition, obtaining high-purity methyl oleate from natural plant oils is a costly process. Thus, development of new plant oil-derived acrylates suitable for PSA applications is necessary.
Acrylic PSAs are generally copolymers of three types of monomers:3,28 low-Tg acrylate (e.g., butyl acrylate, 2-EHA), the soft monomer, which provides softness and tackiness; high-Tg acrylate (e.g., methyl acrylate, vinyl acetate), the hard monomer, which provides polymer cohesive strength; and unsaturated carboxylic acid (e.g., acrylic acid, methyl acrylic acid), the polar monomer, which adjusts polarity and provides cross-linking sites to improve peel and shear strength. Several technologies can be used to develop acrylic PSAs: solvent-based, water-based, hot-melt, and UV (radiation). UV curing is the most environmentally friendly process because it uses no volatile solvents and consumes less heat energy than other techniques.
Acrylic polyol is a compound that contains both vinyl group and multi-hydroxyl functionalities. Free-radically polymerized acrylic polyols (polymerization through vinyl groups) still possess a large number of hydroxyl groups. The polar nature of such polymers is expected to offer good polar attraction to substrates when used as PSAs. The objectives of this study were to synthesize new types of acrylic polyols from epoxidized soybean oils (ESO) and develop UV-cured PSAs. ESO was first partially acrylated via a ring-opening reaction with acrylic acid to obtain AESO. Remaining epoxides of AESO were then di-hydroxylated with water to obtain acrylic polyols, which were further polymerized and investigated for PSA applications. The degree of acrylation was carefully controlled, so the polymers had both sufficient flexibility to possess good wettability and appropriate cross-linking to maintain adequate cohesive strength.
2. Materials and methods
2.1 Materials
ESO (VIKOFLEX 7170; epoxy oxygen content 6.7%, molecular weight 1000 g mol−1) was provided by Arkema Inc. (King of Prussia, PA). AMC-2 (a solution of 40–60% chromium(III) 2-ethylhexanoate in a mixture of di(heptyl, nonyl, undecy) phthalates) was supplied by Ampac Fine Chemicals (Rancho Cordova, CA). Rosin ester (SYLVALITE® RE 80 HP) was obtained from Arizona Chemical (Jacksonville, FL). Darocur 1173 (2-hydroxy-2-methyl-1-phenyl-propan-1-one) was provided by BASF (Florham Park, NJ). Perchloric acid (70 wt% solution in water), tetrahydrofuran, ether, ethyl acetate, anhydrous magnesium sulfate, celite 545, hydroquinone, acrylic acid (99%), 2-ethylhexyl acrylate (2-EHA, 98%), butyl acrylate (>99%), and methyl acrylate (99%) were purchased from Sigma-Aldrich (St. Louis, MO) and Fisher Scientific (Waltham, MA) and used as received. Commercial acrylated epoxidized soybean oil (CAESO, acrylate functionality 2.7, hydroxyl value 156 mg KOH g−1) was obtained from Sigma-Aldrich.2.2 Synthesis of acrylated epoxidized soybean oils (AESO)
Acrylation of ESO was carried out in a 250 mL three-neck flask equipped with a mechanical stirrer in a silicone oil bath according to the reference with some modifications.29 Predetermined molar ratios of acrylic acid to epoxide groups were used to obtain AESO with the necessary acrylate functionality. An excess of 0.1 mol acrylic acid per mol of epoxide groups to be acrylated was used to maximize the level of acrylation. Acrylic acid was added in a number of aliquots throughout the reaction to reduce the extent of epoxy homopolymerization. For a typical reaction of synthesizing AESO with 2 acrylate groups per triglyceride, 100.00 g (0.1 mol) ESO was charged into the flask, then 0.33 g hydroquinone as an inhibitor of acrylate polymerization and 2.00 g AMC-2 as an acrylation catalyst were added. When the reactant temperature reached 80 °C, 7.20 g (0.1 mol) acrylic acid was pipetted into the flask. This amount corresponds to an addition of 1 mol acrylic acid per mol triglyceride. Two hours later, 7.92 g (0.11 mol) was added to the flask, and the reaction continued for another 4 hours. The product was purified through ether extraction and washed with water to eliminate the hydroquinone inhibitor and excess acrylic acid. The organic phase was dried over anhydrous magnesium sulfate then filtrated and concentrated under vacuum to obtain AESO. AESO with 1, 1.5, and 2 acrylate groups per triglyceride was denoted as AESO1, AESO1.5, and AESO2, respectively.2.3 Synthesis of acrylated soybean oil polyols (acrylic polyols, DAESO)
Acrylic polyols were synthesized from AESO by converting the remaining epoxy groups into dihydroxyl groups with water and acid catalysts. For a typical reaction, 50 g AESO was transferred into a Pyrex flask, and 50 mL tetrahydrofuran was added to dissolve the AESO. 25 mL of distilled water and 1.07 mL perchloric acid (70 wt% solution in water) were mixed in another container then dropped into the flask gradually (within 2 min) while stirring. The reaction continued for 5 hours at room temperature. The product was purified through ethyl acetate extraction, washed with distilled water, neutralized with saturated sodium bicarbonate solution, and finally evaporated using the rotary evaporator under vacuum to remove solvent and water. Acrylic polyols from AESO1, AESO1.5, and AESO2 were denoted as DAESO1, DAESO1.5, and DAESO2, respectively.2.4 Characterization of AESO and DAESO
Epoxy content was measured according to ASTM D 1652-97 (Test Method A). Hydroxyl value (OHV) was measured according to ASTM D 4274-99 (Test Method A-Acetylation). 1H nuclear magnetic resonance (1H-NMR) spectra were acquired at room temperature on a Varian VNMRS 600 MHz SB NMR spectrometer in deuterated chloroform (D1 = 3 s, 40 scans). Relative peaks were integrated to quantify the functionality of epoxy and acrylate groups. Fourier transform infrared spectroscopy (FTIR) spectra were acquired with a PerkinElmer Spectrum 400 FT-IR/FT-NIR Spectrometer (Waltham, MA). Spectra were collected in the region of 4000 to 400 cm−1 with a spectral resolution of 4 cm−1, and 32 scans were co-added.2.5 UV polymerization of acrylic polyols
DAESO was mixed with 2-EHA (soft monomer), acrylic acid (polar monomer), and the photoinitiator according to formulas shown in Table 1 to obtain rosin-free polymers (S1–S7). The mixture was thoroughly mixed in a 25 mL glass vial with the aid of a Vortex mixer and sonicator, then spread onto release paper. The resin was free-radically polymerized with a Fusion 300S 6′′ UV system (300 W per inch power, D bulb, UVA radiation dose 215–231 mJ cm−2) equipped with an LC6B bench-top conveyor at conveyor speed of 7 ft min−1 for 1 pass. The cured specimen was peeled off the release paper and stored for characterization. The ratio of DAESO to 2-EHA and acrylate functionality was varied in different formulas to study their effects on polymer properties. Formula with AESO1 was prepared as a comparison to DAESO1 formulation, so that we could study how the introduction of extra hydroxyl groups onto triglycerides related to polymer properties. Furthermore, we also prepared polymers from CAESO to compare with our synthesized acrylic polyols.| Samplea | DAESO1, g | DAESO1.5, g | DAESO2, g | CAESO, g | AESO1, g | 2-EHA, g | Samplea,b |
|---|---|---|---|---|---|---|---|
| a Each formula also contains a fixed amount of 0.1 g acrylic acid and 0.06 g photoinitiator.b PS sample composition is similar to the corresponding S sample, except that the PS formula also contains 0.5 g rosin ester. | |||||||
| S1 | 0.75 | — | — | — | — | 1.15 | PS1 |
| S2 | 1 | — | — | — | — | 0.9 | PS2 |
| S3 | 1.25 | — | — | — | — | 0.65 | PS3 |
| S4 | — | 1 | — | — | — | 0.9 | PS4 |
| S5 | — | — | 1 | — | — | 0.9 | PS5 |
| S6 | — | — | — | 1 | — | 0.9 | PS6 |
| S7 | — | — | — | — | 1 | 0.9 | PS7 |
We further prepared PSAs according to formulas shown in Table 1 (PS1–PS7). The composition of PSA resins was similar to S1–S7, except that 0.5 g rosin ester was added to each formula. For a typical PSA sample preparation, the ingredients were mixed in a glass vial then coated onto PET film using an EC-200 Drawdown Coater with a #20 wire bar (Chem Instruments Inc., Fairfield, OH). The coating amount was calculated to be 48.55 g m−2, and wet thickness was 50 microns. The adhesive coating was cured similarly as above and stored for further evaluation. Non-supported PSAs were also prepared for characterization purposes.
2.6 Characterization of polymers and PSAs
Thermal transitions of the polymers were measured with a TA Q200 differential scanning calorimetry (DSC) instrument in an inert environment using nitrogen with a gas flow rate of 50 mL min−1. About 10 mg sample was sealed in a stainless steel pan. An empty pan was used as a reference. The sample was heated from −90 °C to 100 °C at a rate of 10 °C min−1. Phase transition temperatures, including glass transition (Tg), melting (Tm), heat capacity (ΔCp), and heat of melting (ΔHm) were obtained.Dynamic mechanical analysis (DMA) of polymers was conducted using a TA Q800 DMA analyzer equipped with a liquid nitrogen cooling system in a shear sandwich mode at 1 Hz frequency and 0.1% strain (linear viscoelastic region). Specimens (about 10 mm × 10 mm × 1 mm) were cut from a cured polymer sheet with a blade and heated from −120 to 120 °C at a rate of 3 °C min−1 under a nitrogen atmosphere. Storage modulus (G′), loss modulus (G′′), and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ were obtained.
δ were obtained.
Gel content was measured by immersing the sample in a large amount of toluene.30 Approximately 0.2 g of polymer film was accurately weighed and immersed in 20 mL toluene for 1 week. The specimen was then taken out and dried at 130 °C for at least 2 hours until a constant weight was achieved. Gel content was measured according eqn (1) below:
| Gel content = w1/w0 × 100 | (1) |
To determine the mechanical performance of PSAs, the PET films coated with adhesive layers were cut into 1-inch × 5-inch stripes. Peel strength was measured following ASTM D3330/D3330M-04(2010), and loop tack strength was measured following ASTM D6195-03(2011) using IMADA MV-110-S tester (Imada Inc., Northbrook, IL) on 18-gauge, 304 stainless steel test panels (ChemInstruments, Inc., Fairfield, OH) with a stressing clamp moving speed of 5.0 mm s−1. Five specimens were measured for each formula. The shear test was conducted following ASTM D3654/D3654M-06 (2011) using Room Temperature 10 Bank Shear (ChemInstruments, Inc., Fairfield, OH) with a specimen size of 1 inch × 1 inch and test mass of 1000 g on 18-gauge, 304 stainless steel test panels. The time between the application of the load to the specimen and its separation from the panel was recorded.
Viscoelasticity of PSAs was measured using a Bohlin CVOR 150 rheometer (Malvern Instruments, Southborough, MA) with a PP 8 parallel plate. The specimen was cut from non-supported PSA sheets using a lab-made cutter and placed between the two parallel plates. The gap was closed with a set normal force. The strain amplitude was set at 0.1% (within linear viscoelastic region), and an oscillatory frequency sweep was performed from 0.01 to 100 rad s−1. Storage modulus (G′) and loss modulus (G′′) as a function of frequency were recorded to show how the adhesives responded at different time scales.
3. Results and discussion
3.1 Acrylic polyols
Acrylate functionality was grafted onto triglycerides through the reaction of epoxide ring of ESO with acrylic acid. Converting the epoxy to vinyl ester enables the resin to be free-radically polymerized. The ring-opening reaction of the remaining epoxy groups with water results in acrylic polyols (Fig. 1). | ||
| Fig. 1 Synthesis of acrylic polyols from ESO (functionality of acrylate, epoxide, and hydroxyl groups of triglycerides may vary depending on reaction conditions). | ||
The conversion of ESO to AESO then to DAESO was confirmed with FTIR (Fig. 2). ESO exhibited a characteristic epoxide peak at 822 and 842 cm−1 (inset of Fig. 2). Compared with the spectrum of ESO, the intensity of the epoxide peak was reduced in AESO1 due to the conversion of one epoxy group to one acrylate and one hydroxyl. The acrylation was evidenced by the new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching peaks of the acrylate group at 1635, 1618, and 810 cm−1 and the hydroxyl peak at 3450 cm−1. No epoxy peak was observed for DAESO1, whereas the intensity of hydroxyl peak at 3450 cm−1 increased greatly, indicating that all the remaining epoxy groups of AESO1 reacted and were mostly or completely converted into hydroxyls. A small shoulder was observed at 1067 cm−1, which was caused by the side reaction of epoxy homopolymerization-generating ether groups.
C stretching peaks of the acrylate group at 1635, 1618, and 810 cm−1 and the hydroxyl peak at 3450 cm−1. No epoxy peak was observed for DAESO1, whereas the intensity of hydroxyl peak at 3450 cm−1 increased greatly, indicating that all the remaining epoxy groups of AESO1 reacted and were mostly or completely converted into hydroxyls. A small shoulder was observed at 1067 cm−1, which was caused by the side reaction of epoxy homopolymerization-generating ether groups.
NMR is another powerful tool to confirm the reaction of triglycerides modification, as well as to quantify the functionality of relative groups (e.g., epoxy, acrylate). Typical spectra of ESO, AESO, and DAESO were presented, and peak assignments of triglyceride protons were noted (Fig. 3). The two epoxide protons (peak 3) appeared at 2.8–3.2 ppm in ESO, decreased in area in AESO1, and disappeared in DAESO1. On the other side, three sets of new peaks appeared at 5.7–6.7 ppm in AESO and DAESO, representing the three protons attached to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of acrylate groups (peak 11a–c).31 Using methyl protons (0.9–0.98 ppm, peak 8) or an α-methylene proton (2.3 ppm, peak 4) as an internal standard, the functionalities of epoxy and acrylate per triglycerides were calculated (Table 2).32 The acrylate functionality based on NMR was similar to the theoretical functionality based on the amount of acrylic acid addition during synthesis. Protons germinal to hydroxyl groups (peak 12) appeared at 3.26–4.25 ppm; however, these peaks overlapped with the peak of glycerol methylene protons (peak 2, 4.1–4.3 ppm) and the peak of protons vicinal and germinal to possible ether linkages32 caused by epoxy homopolymerization during acrylation and dihydroxylation. Therefore, the relative hydroxyl functionality of resins was evaluated through their respective hydroxyl value (OHV). The OHV of AESO1 was 107.8 mg KOH g−1. More hydroxyl groups were introduced into the triglyceride structure via a dihydroxylation reaction, the OHV of DAESO1 was 260.9, and that of DAESO1.5 and DAESO2 was 255 and 212 mg KOH g−1, respectively. The epoxy content of acrylic polyols measured through a titration approach was nearly 0% (Table 2), which was consistent with NMR measurements.
C double bond of acrylate groups (peak 11a–c).31 Using methyl protons (0.9–0.98 ppm, peak 8) or an α-methylene proton (2.3 ppm, peak 4) as an internal standard, the functionalities of epoxy and acrylate per triglycerides were calculated (Table 2).32 The acrylate functionality based on NMR was similar to the theoretical functionality based on the amount of acrylic acid addition during synthesis. Protons germinal to hydroxyl groups (peak 12) appeared at 3.26–4.25 ppm; however, these peaks overlapped with the peak of glycerol methylene protons (peak 2, 4.1–4.3 ppm) and the peak of protons vicinal and germinal to possible ether linkages32 caused by epoxy homopolymerization during acrylation and dihydroxylation. Therefore, the relative hydroxyl functionality of resins was evaluated through their respective hydroxyl value (OHV). The OHV of AESO1 was 107.8 mg KOH g−1. More hydroxyl groups were introduced into the triglyceride structure via a dihydroxylation reaction, the OHV of DAESO1 was 260.9, and that of DAESO1.5 and DAESO2 was 255 and 212 mg KOH g−1, respectively. The epoxy content of acrylic polyols measured through a titration approach was nearly 0% (Table 2), which was consistent with NMR measurements.
| Samplea | Epoxy content, % | Hydroxyl value, mg KOH g−1 | Epoxy functionality-NMR | Acrylate functionality-NMR | Theoretical acrylate functionality |
|---|---|---|---|---|---|
| a AESO1 stands for acrylated epoxidized soybean oil with 1 acrylate group per triglyceride; DAESO1, DAESO1.5, and DAESO2 stand for acrylic polyols with 1, 1.5, and 2 acrylate groups per triglyceride, respectively; CAESO stands for commercial acrylated epoxidized soybean oil. | |||||
| AESO1 | 4.04 ± 0.07 | 107.8 ± 7.0 | 3.7 | 1.1 | 1.0 |
| DAESO1 | 0.13 ± 0.03 | 260.9 ± 5.5 | 0 | 1.1 | 1.0 |
| DAESO1.5 | 0.09 ± 0.01 | 255.0 ± 6.1 | 0 | 1.5 | 1.5 |
| DAESO2 | 0.12 ± 0.02 | 212.0 ± 6.5 | 0 | 2.0 | 2.0 |
| CAESO | 0.11 ± 0.02 | 156.0 ± 3.4 | 0 | 2.7 | — |
3.2 FTIR and thermal properties of acrylic polyol polymers
When liquid acrylate resins were exposed to UV radiation in the presence of a photoinitiator, free-radical polymerization took place rapidly and led to polymer networks. Typical FTIR spectra of acrylate resins (S2 from Table 1) before and after UV radiation were presented in Fig. 4. The completeness of polymerization was confirmed by the disappearance of several acrylate peaks: 1635, 1618, 1406, 984, and 810 cm−1.Thermal properties of the monomers and polymers confirmed that DAESO1 was a hard monomer and 2-EHA was a soft monomer (Table 3 and Fig. S1†). The Tg of DAESO1 and its homopolymer were −31.3 °C and −27.2 °C, respectively, and Tg of 2-EHA and its homopolymer were −85 °C and −58 °C. With the increase of the amount of DAESO1 in the resin from 0.75 to 1 then to 1.25 g (S1 vs. S2 vs. S3, see formulation in Table 1), the Tg of polymers increased from −46.4 to −41.6 then to −35.2 °C, and Tm also increased slightly from −0.1 to 4 °C. Since DAESO1 was a relatively hard monomer, it is reasonable that polymer Tg increased and flexibility decreased as more acrylic polyol was added to the resin formulation.
| Sample | Tg, °C | ΔCp, J (g−1 °C−1) | Tm, °C | ΔHm, J g−1 |
|---|---|---|---|---|
| DAESO1 | −31.3 | 0.36 | 7.4 | 13.1 |
| 2-EHA | −85 | — | — | — |
| DAESO1 homopolymer | −27.2 | 0.41 | 9.8 | 10.3 |
| 2-EHA homopolymer | −58 | — | — | — |
| S1 | −46.4 | 0.18 | −0.1 | 2.9 |
| S2 | −41.6 | 0.27 | 2.7 | 3.6 |
| S3 | −35.2 | 0.30 | 4.0 | 4.8 |
| S4 | −38.9 | 0.37 | 4.7 | 1.5 |
| S5 | −30.5 | 0.50 | — | — |
| S6 | −22.5 | 0.51 | — | — |
| S7 | −59.5 | 0.37 | −9.5 | 4.1 |
We further studied the effects of acrylate functionality on the Tg of polymers. As the acrylate functionality increased from 1 to 1.5 to 2, Tg of the polymers increased from −41.6 to −38.9 then to −30.5 °C (S2 vs. S4 vs. S5, Table 3), indicating higher functionality leading to higher degree of cross-linking, thus higher Tg and less flexibility.33 This is also why S6, the sample based on commercial AESO with acrylate functionality of 2.7, had the highest Tg of −22.5 °C (Table 3). Although the acrylate functionality of DAESO1 was the same as AESO1 (Table 2), the hydroxyl functionality of DAESO1 is much larger than AESO1 (261 vs. 108 mg KOH g−1). As a result, more hydrogen bonding was formed within S2 than in S7, resulting in higher Tg of S2.34,35
3.3 Dynamic mechanical properties of acrylic polyol polymers
Storage modulus (G′) and damping factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) of the polymers as a function of temperature were shown in Fig. 5. Tg,DMA of polymers was taken as the peak temperature of tan
δ) of the polymers as a function of temperature were shown in Fig. 5. Tg,DMA of polymers was taken as the peak temperature of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, which is larger than the respective Tg from DSC but changes in the same trend according to polymer composition. The differences between Tg,DMA and Tg from DSC are normal, because Tg,DMA was obtained based on the measurement of mechanical strength and energy loss at 3 °C min−1 heating rate and 1 Hz, whereas Tg from DSC was obtained based on heat flow (i.e., heat capacity) measurement at heating rate of 10 °C min−1.36
δ, which is larger than the respective Tg from DSC but changes in the same trend according to polymer composition. The differences between Tg,DMA and Tg from DSC are normal, because Tg,DMA was obtained based on the measurement of mechanical strength and energy loss at 3 °C min−1 heating rate and 1 Hz, whereas Tg from DSC was obtained based on heat flow (i.e., heat capacity) measurement at heating rate of 10 °C min−1.36
The height of peak tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (tan
δ (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δpeak height) varied according to polymer compositions (Table 4). tan
δpeak height) varied according to polymer compositions (Table 4). tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δpeak height decreased as acrylate functionality increased, with S6 of the lowest value of 0.37 and S7 of the highest value of 0.83. Polymers with larger tan
δpeak height decreased as acrylate functionality increased, with S6 of the lowest value of 0.37 and S7 of the highest value of 0.83. Polymers with larger tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δpeak height have better capacity for energy dissipation, which benefits applications such as PSAs. All the seven polymers exhibited a clear rubbery plateau region, indicating that these polymers were cross-linked. With larger amount (S1 vs. S2 vs. S3) or higher acrylate functionality (S2 vs. S4 vs. S5 vs. S6) of acrylic polyols in the composition, G′ of polymer was higher at all temperatures except for a few fluctuations in the glassy region. To better under DMA properties, the experimental cross-link density (νe) of the polymer was determined from the rubbery plateau modulus according to eqn (2):37
δpeak height have better capacity for energy dissipation, which benefits applications such as PSAs. All the seven polymers exhibited a clear rubbery plateau region, indicating that these polymers were cross-linked. With larger amount (S1 vs. S2 vs. S3) or higher acrylate functionality (S2 vs. S4 vs. S5 vs. S6) of acrylic polyols in the composition, G′ of polymer was higher at all temperatures except for a few fluctuations in the glassy region. To better under DMA properties, the experimental cross-link density (νe) of the polymer was determined from the rubbery plateau modulus according to eqn (2):37
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak temperature (Tg,DMA), R is the gas constant, and T is the corresponding absolute temperature (Tg,DMA + 50). Molecular weight between cross-links (Mc) was calculated according to eqn (3):
δ peak temperature (Tg,DMA), R is the gas constant, and T is the corresponding absolute temperature (Tg,DMA + 50). Molecular weight between cross-links (Mc) was calculated according to eqn (3):
 | (3) |
| Sample | Density, g cm−3 | Tg,DMA, °C | tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δpeak height δpeak height |
G′ @ Tg,DMA + 50, MPa | Tg,DMA + 50, °C | Cross-link density, νe, mol m−3 | Mc, g mol−1 | Gel content, % |
|---|---|---|---|---|---|---|---|---|
| a Prerequisite Mc for PSA is 104–105 g mol−1.38 | ||||||||
| S1 | 1.26 | 0.1 | 0.76 | 0.07 | 50.1 | 26.0 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375.0 375.0 |
73.25 ± 0.33 |
| S2 | 1.23 | 5.0 | 0.76 | 0.10 | 55 | 36.7 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 557.3 557.3 |
74.90 ± 0.40 |
| S3 | 1.26 | 12.4 | 0.70 | 0.16 | 62.4 | 57.4 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 969.4 969.4 |
75.86 ± 0.41 |
| S4 | 1.13 | 6.0 | 0.66 | 0.23 | 56 | 84.0 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444.8 444.8 |
81.72 ± 0.43 |
| S5 | 1.11 | 9.7 | 0.54 | 0.29 | 59.7 | 104.8 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 592.1 592.1 |
87.55 ± 0.22 |
| S6 | 1.08 | 20.1 | 0.37 | 0.65 | 70.1 | 227.8 | 4741.7 | 93.32 ± 0.22 |
| S7 | 1.27 | −19.8 | 0.83 | 0.09 | 30.2 | 35.7 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 589.0 589.0 |
70.62 ± 0.37 |
| PSA prerequisitea | 104–105 | |||||||
Rubbery plateau modulus and cross-link density increased, whereas molecular weight between cross-links decreased as the amount of DAESO1 acrylic polyol in the resin increased from 0.75 to 1 then to 1.25 g refer to 2-EHA (S1 vs. S2 vs. S3) (Table 4). As a result, the S1 polymer should have little cohesive strength and therefore poor shear properties; however, it easily wet the substrate to achieve good initial tack strength when used as PSA. Acrylic polyol acted as both the polymer backbone and cross-linker, whereas 2-EHA and acrylic acid were linear chain extenders to modify the polymer flexibility and polarity and did not contribute to cross-linking. It is noteworthy to mention that although DAESO1 averaged only 1.1 acrylate per triglyceride based on 1H-NMR analysis, the factual acrylate functionality of the triglyceride is larger than 1.1, because soybean oil triglycerides also contain 16.1% saturated fatty acids (C14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 16
0, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, and 18
0, and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0). These fatty acids/triglycerides could not be functionalized and polymerized and acted as a plasticizer for the polymers.
0). These fatty acids/triglycerides could not be functionalized and polymerized and acted as a plasticizer for the polymers.
We also observed that the acrylate functionality had positive effects on rubbery plateau modulus and cross-link density of the polymers (Table 4, samples S2 vs. S4 vs. S5 vs. S6). Higher acrylate functionality created a more cross-linked structure during polymerization, thus a higher modulus. The rubbery plateau modulus, cross-link density, and molecular weight between cross-links are similar for S2 and S7, because AESO1 and DAESO1 had the same acrylate functionality. The molecular weight between cross-links (Mc) of all the polymers met the prerequisite for PSAs except for S6 (with Mc of 4742 g mol−1). The Mc value for PSAs needs to be about 104–105 g mol−1 in order to achieve higher peel energy due to fibrillation during the peeling process.38
Polymers formulated with higher acrylate functionality, such as S6 and S5, displayed higher gel contents than the polymer with lower acrylate functionality (i.e., S1–S3, S4, S7) (Table 4). Gel content generally correlated well with the rubbery plateau modulus and cross-link density,30 which is also observed in this study.
3.4 Adhesive performance of PSAs
PSA composition was similar to the corresponding polymer discussed above (Table 1), except that the same amount of rosin was added to each formulation to adjust viscoelastic properties and meanwhile improve adhesion performances. Tack, peel, and shear resistance are three fundamental and interconnected adhesion properties of PSAs.4 Tack value shows the ability of adhesives to wet the substrate instantaneously, peel strength indicates adhesion to the substrate, and shear resistance reveals the cohesion strength of the polymers as adhesives. All PSAs exhibited excellent shear resistance (>50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 min), except for PS1 and PS7 (Table 5). As discussed previously, the polymers of PS2, PS3, PS4, PS5, and PS6 have larger rubbery plateau moduli and crosslink densities than PS1 and PS7 polymers, thus stronger polymer cohesive strengths. The shear resistance of PS1 (5000 min) was larger than PS7 (500 min) because the polymer of PS1 had more hydroxyl groups than that of PS7 in forming physical entanglement through hydrogen bonding. Such physical entanglements contributed to cohesive strength.
000 min), except for PS1 and PS7 (Table 5). As discussed previously, the polymers of PS2, PS3, PS4, PS5, and PS6 have larger rubbery plateau moduli and crosslink densities than PS1 and PS7 polymers, thus stronger polymer cohesive strengths. The shear resistance of PS1 (5000 min) was larger than PS7 (500 min) because the polymer of PS1 had more hydroxyl groups than that of PS7 in forming physical entanglement through hydrogen bonding. Such physical entanglements contributed to cohesive strength.
| Sample | Peel strength, N in−1 | Tack, N in−1 | Shear resistance, min |
|---|---|---|---|
| a Some cohesive failure was noticed.b Peel strength of S2 pure polymer (without rosin) was 0.76 ± 0.06 N in−1. | |||
| PS1 | 4.29 ± 0.17a | 8.16 ± 0.21 | 5300 ± 980 |
| PS2b | 4.47 ± 0.13 | 7.14 ± 0.05 | >50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| PS3 | 2.87 ± 0.60 | 4.75 ± 0.95 | >50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| PS4 | 2.42 ± 0.51 | 2.03 ± 0.25 | >50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| PS5 | 0.77 ± 0.27 | 0.47 ± 0.21 | >50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| PS6 | 0.70 ± 0.13 | 0.08 ± 0.01 | >50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| PS7 | 1.15 ± 0.09 | 1.56 ± 0.02 | 500 ± 100 |
PS1 had peel strength of 4.29 N in−1 and tack of 8.16 N in−1, but slight cohesive failure (i.e., adhesive residue remained on substrates after peeling) was observed (Table 5). This is because the polymer backbone was soft and weak due to a large amount of soft monomer (i.e., 2-EHA) in the formulation, and did not form enough crosslinking. By increasing the amount of DAESO1 in the composition, which acted as both a hard monomer and a cross-linker, PS2 had a good balance of peel (4.47 N in−1), tack (7.14 N in−1), and shear (>50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 min) performance. The peel adhesion strength was higher than that of commercial Scotch® transparent tape (2.45 N in−1).39 Further increasing DAESO1 led to decreased peel and tack properties (i.e., PS3 Table 5), due to less flexibility to wet the adherend sufficiently and form effective bonds, which corresponds to the relatively high glass transition temperature (Tg of −35.2 °C from DSC and Tg,DMA of 12.4 °C) and large crosslink density (57.4 mol m−3) of the S3 polymer.
000 min) performance. The peel adhesion strength was higher than that of commercial Scotch® transparent tape (2.45 N in−1).39 Further increasing DAESO1 led to decreased peel and tack properties (i.e., PS3 Table 5), due to less flexibility to wet the adherend sufficiently and form effective bonds, which corresponds to the relatively high glass transition temperature (Tg of −35.2 °C from DSC and Tg,DMA of 12.4 °C) and large crosslink density (57.4 mol m−3) of the S3 polymer.
PS2, PS4, PS5, and PS6 PSAs were based on the same amount of acrylic polyols with 1.1, 1.5, 2, and 2.7 acrylates functionality, respectively. Increasing the functionality of acrylic polyols significantly reduced PSA peel and tack values (Table 5). For example, the peel and tack values of PS2 were 4.47 and 7.14 N in−1, whereas those of PS6 was only 0.70 and 0.08 N in−1. Several reasons attributed to this: first, polymers with high functionality acrylic polyols were highly cross-linked resulted in insufficient flexibility to wetting the substrate and forming bonds; second, high functionality acrylic polyols had less polar hydroxyl groups (i.e., low hydroxyl values). The hydroxyl groups of these polymers acted as a critical adhesion site to improve wetting onto the stainless steel testing panel and accelerate the rate of bond establishment and development via the formation of hydrogen bonding and other noncovalent interactions.
PS2 had much better PSA performance than PS7 (Table 5), even though they were polymerized with the same amount of monomers with identical acrylate functionality (1.1). As mentioned before, DAESO1 of PS2 (OHV of 261 mg KOH g−1) had more hydroxyl groups than AESO1 of PS7 (OHV of 108 mg KOH g−1). This result further confirms that building extra hydroxyl groups into acrylated triglycerides is critical to developing PSAs in soybean oil system.
The effects of co-monomer type, acrylic acid amount, acrylic polyol to 2-EHA ratio, and rosin amount on PSA adhesion strength were shown in the ESI (Table S1–S4†). In this study, 2-EHA was selected as soft co-monomer against butyl acrylate (BA) and methyl acrylate (MA), because both BA and MA produced rigid polymers (ESI Table S1†). We used acrylic acid (AA) against methyl acrylic acid (MAA), because MAA formulation led to PSA with 100% cohesive failure (ESI Table S1†). Increasing the amount of acrylic acid from 0 g to 0.1 g then to 0.2 g first increased then decreased peel adhesion (ESI Table S2†). An appropriate amount of acrylic acid improved the polarity of the adhesive, resulting in better adhesion; however, excess acrylic acid would decrease polymer flexibility. Increasing the amount of rosin ester in the formulation also led to better adhesion, but when it exceeded a critical amount, adhesive cohesive strength decreased and cohesive failure occurred (ESI Table S4†).
3.5 PSA viscoelasticity
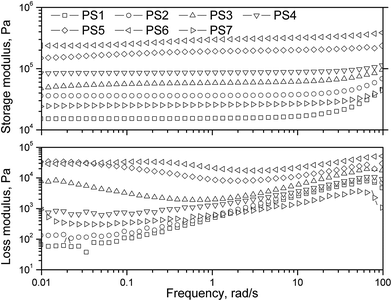
Mechanical performances of PSAs are closely related to their viscoelastic properties. According to Dahlquist's criterion, a PSA needs to have a plateau modulus lower than 3.3 × 105 Pa so it can wet the substrate and exhibit tack during bonding.40,41 Correlations between tack, shear and peel adhesion behaviors and viscoelastic responses have been well established.3,42 The viscoelastic information at low frequency (∼0.01 rad s−1) describes the bond formation, whereas that at high frequency (∼100 rad s−1) describes the behavior of debonding.43 Therefore, a PSA with good performance should have a low bonding plateau modulus at the bonding frequency (i.e., low G′ at 0.01 rad s−1) and high energy dissipation at the debonding frequency (i.e., high G′′ at 100 rad s−1).Frequency sweep spectra of these PSAs are presented in Fig. 6, and characteristic viscoelastic values are summarized in Table 6. The Dahlquist criterion is an important reference because it implies whether a material would be contact efficient (PSA) or deficient (non PSA). Because of the subambient Tg of the PSAs, G′ at 0.01 rad s−1 indicates the value of the plateau modulus.43 The plateau modulus of all the samples except for PS5 and PS6 were much below 3.3 × 105 Pa (Dahlquist's criterion), which met the prerequisite for PSAs. The plateau modulus of PS5 and PS6 were very close to the limit, implying their contact deficiency. Peel performance is dependent upon both bonding efficiency (G′ at 0.01 rad s−1) and debonding resistance (G′ and G′′ at 100 rad s−1).43 The lower the G′(0.01 rad s−1), the more favorable the bonding and the higher the peel strength. Furthermore, G′ at 100 rad s−1 indicates the cohesive strength of adhesive, and G′′ at 100 rad s−1 shows the energy of dissipation. Therefore, the higher G′ (100 rad s−1) and G′′ (100 rad s−1), the higher the peel strength.
| Sample | G′ (0.01 rad s−1), Pa | G′′ (0.01 rad s−1), Pa | G′ (1 rad s−1), Pa | G′ (100 rad s−1), Pa | G′′ (100 rad s−1), Pa |
|---|---|---|---|---|---|
| a According to the Dahlquist criterion, the prerequisite plateau modulus value (G′ at 0.01 rad s−1) for PSA should be lower than 3.3 × 105 Pa.40,41 | |||||
| PS1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
66.8 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 460 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
4759 |
| PS2 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
133.9 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
7032 |
| PS3 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 910 |
7875 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
| PS4 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
978 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
113![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
9266 |
| PS5 | 148![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 830 |
192![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
237![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 510 |
| PS6 | 238![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
386![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 960 |
| PS7 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
913.3 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 690 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
1075 |
| Dahlquista | <3.3 × 105 | ||||
PS2 had a good balance of relatively low G′ (0.01 rad s−1) of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 Pa and high G′ (100 rad s−1) and G′′ (100 rad s−1) of 68
200 Pa and high G′ (100 rad s−1) and G′′ (100 rad s−1) of 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Pa and 7032 Pa, corresponding to its high peel strength of 4.47 N in−1, while the G′(0.01 rad s−1) values of PS3, PS4, PS5, and PS6 were all much higher than that of PS2, which limited bonding efficiency and were consistent with lower peel values. Similar to peel correlation, tack performance also depends on bonding efficiency and debonding resistance (G′ and G′′ at 100 rad s−1), except that the bonding frequency during tack measurement was about 1 rad s−1, rather than 0.01 rad s−1.43 Comparing the tack values in Table 5 and G′(1 rad s−1), G′ (100 rad s−1), and G′′ (100 rad s−1) in Table 6 shows that they were also well correlated. Shear performance was correlated with the G′ at 0.01 rad s−1.43 Generally, the higher the G′ (0.01 rad s−1), the better the shear. G′ (0.01 rad s−1) of PS1 and PS7 was smaller than other PSAs, which corresponded to their weak shear resistance.
600 Pa and 7032 Pa, corresponding to its high peel strength of 4.47 N in−1, while the G′(0.01 rad s−1) values of PS3, PS4, PS5, and PS6 were all much higher than that of PS2, which limited bonding efficiency and were consistent with lower peel values. Similar to peel correlation, tack performance also depends on bonding efficiency and debonding resistance (G′ and G′′ at 100 rad s−1), except that the bonding frequency during tack measurement was about 1 rad s−1, rather than 0.01 rad s−1.43 Comparing the tack values in Table 5 and G′(1 rad s−1), G′ (100 rad s−1), and G′′ (100 rad s−1) in Table 6 shows that they were also well correlated. Shear performance was correlated with the G′ at 0.01 rad s−1.43 Generally, the higher the G′ (0.01 rad s−1), the better the shear. G′ (0.01 rad s−1) of PS1 and PS7 was smaller than other PSAs, which corresponded to their weak shear resistance.
We further comparatively studied the viscoelasticity of S2 and PS2 of pure polymers and formulated PSAs containing rosin ester tackifier (Fig. 7). Tackifier is usually a critical composition of PSA to balance the viscoelastic property of the adhesive suitable for bonding and debonding.44 It is obvious that plateau modulus (G′ at 0.01 rad s−1) was greatly reduced by adding rosin tackifier, which ensured a good wetting and bonding of PS2 at bonding frequency. Moreover, G′ and G′′ at 100 rad s−1 were still high enough to achieve adequate cohesive strength and energy dissipation. The viscoelasticity information was consistent with the significantly higher peel adhesion strength of PS2 (4.47 N in−1) compared with that of S2 (0.76 N in−1). A similar tackifier effect on the viscoelastic properties of polyolefin based PSAs was also reported.45
4. Conclusions
Acrylic polyols with 1, 1.5, and 2 acrylates per triglycerides were synthesized, and viscoelastic acrylate polymers were developed via UV polymerization. DSC and DMA measurements revealed that polymers based on a moderate amount of acrylic polyol with lower acrylate functionality (∼1) had a better balance of wettability (i.e., Tg) and cohesive strength (i.e., rubbery plateau modulus or cross-link density) and thus were more suitable for PSAs. Compared with polymers from commercial fully acrylated epoxidized soybean oil, the semi-acrylic polyols provide the most potential for PSA applications. This was attributed to the extra polar hydroxyl groups on the polymer backbones, which act as critical adhesion sites to improve wetting onto the substrate and accelerate bonding via polar attraction. The newly developed biobased acrylic polyols are promising alternatives to petrochemical based counterparts for acrylic PSAs.Acknowledgements
This is contribution no. 15-117-J from the Kansas Agricultural Experimental Station. Financial support was provided by the USDA-NIFA Biomass Research and Development Initiative program (Grant no. 2012-10006-20230).References
- J. Johnston, Pressure sensitive adhesive tapes: A guide to their function, design, manufacture, and use, Pressure Sensitive Tape Council, Northbrook, IL, 2003 Search PubMed.
- M. F. Tse and L. Jacob, J. Adhes., 1996, 56, 79–95 CrossRef CAS PubMed.
- D. Satas, Handbook of Pressure Sensitive Adhesive Technology, Satas & Associates, 1999 Search PubMed.
- Z. Czech, J. Adhes. Sci. Technol., 2007, 21, 625–635 CrossRef CAS PubMed.
- R. L. Shogren, Z. Petrovic, Z. S. Liu and S. Z. Erhan, J. Polym. Environ., 2004, 12, 173–178 CrossRef CAS.
- M. Alpaslan and H. Gunduz, Food Nahrung, 2000, 44, 434–437 CrossRef CAS.
- V. Sharma and P. P. Kundu, Prog. Polym. Sci., 2006, 31, 983–1008 CrossRef CAS PubMed.
- M. Desroches, M. Escouvois, R. Auvergne, S. Caillol and B. Boutevin, Polym. Rev., 2012, 52, 38–79 CrossRef CAS PubMed.
- Y. Xia and R. C. Larock, Green Chem., 2010, 12, 1893–1909 RSC.
- G. Lligadas, J. C. Ronda, M. Galià and V. Cádiz, Mater. Today, 2013, 16, 337–343 CrossRef CAS PubMed.
- M. A. R. Meier, J. O. Metzger and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1788–1802 RSC.
- J. C. Mol, Green Chem., 2002, 4, 5–13 RSC.
- A. Campanella, M. A. Baltanas, M. C. Capel-Sanchez, J. M. Campos-Martin and J. L. G. Fierro, Green Chem., 2004, 6, 330–334 RSC.
- M. Baehr and R. Muelhaupt, Green Chem., 2012, 14, 483–489 RSC.
- S. Bunker, C. Staller, N. Willenbacher and R. Wool, Int. J. Adhes. Adhes., 2003, 23, 29–38 CrossRef CAS.
- A. Li and K. Li, RSC Adv., 2014, 4, 21521–21530 RSC.
- C. A. Koch, P. Mallya and C. R. Williams, Pressure sensitive adhesives based on renewable resources and related methods, US Patents US20120059087 A1, 2012.
- C. A. Koch, Pressure Sensitive Adhesives Made From Renewable Resources and Related Methods, US Patent US20100261806 A1, 2010.
- B. K. Ahn, S. Kraft, D. Wang and X. S. Sun, Biomacromolecules, 2011, 12, 1839–1843 CrossRef CAS PubMed.
- Y. Li and X. Sun, J. Am. Oil Chem. Soc., 2014, 91, 1425–1432 CrossRef CAS.
- H. Pelletier and A. Gandini, Eur. J. Lipid Sci. Technol., 2006, 108, 411–420 CrossRef CAS PubMed.
- E. Sharmin, S. M. Ashraf and S. Ahmad, Eur. J. Lipid Sci. Technol., 2007, 109, 134–146 CrossRef CAS PubMed.
- J. La Scala and R. P. Wool, J. Am. Oil Chem. Soc., 2002, 79, 59–63 CrossRef CAS PubMed.
- S. B. David, K. Sathiyalekshmi and G. A. G. Raj, J. Mater. Sci.: Mater. Med., 2009, 20, 61–70 CrossRef PubMed.
- B. K. Ahn, J. Sung, N. Rahmani, G. Wang, N. Kim, K. Lease and X. S. Sun, J. Adhes., 2013, 89, 323–338 CrossRef CAS PubMed.
- S. P. Bunker and R. P. Wool, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 451–458 CrossRef CAS PubMed.
- C. M. Klapperich, C. L. Noack, J. D. Kaufman, L. Zhu, L. Bonnaillie and R. P. Wool, J. Biomed. Mater. Res., Part A, 2009, 91, 378–384 CrossRef PubMed.
- Z. Czech and A. Butwin, J. Adhes. Sci. Technol., 2009, 23, 1689–1707 CrossRef CAS PubMed.
- J. La Scala and R. P. Wool, Polymer, 2005, 46, 61–69 CrossRef CAS PubMed.
- R. Vendamme and W. Eevers, Macromolecules, 2013, 46, 3395–3405 CrossRef CAS.
- S. N. Khot, J. J. Lascala, E. Can, S. S. Morye, G. I. Williams, G. R. Palmese, S. H. Kusefoglu and R. P. Wool, J. Appl. Polym. Sci., 2001, 82, 703–723 CrossRef CAS PubMed.
- J. J. L. Scala, PhD, University of Delaware, 2002 Search PubMed.
- J. Bicerano, R. L. Sammler, C. J. Carriere and J. T. Seitz, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 2247–2259 CrossRef CAS.
- Q.-H. Zhou, M. Li, P. Yang and Y. Gu, Macromol. Theory Simul., 2013, 22, 107–114 CrossRef CAS PubMed.
- H. Xu, S.-W. Kuo, J.-S. Lee and F.-C. Chang, Polymer, 2002, 43, 5117–5124 CrossRef CAS.
- C. A. Gracia-Fernández, S. Gómez-Barreiro, J. López-Beceiro, J. Tarrío Saavedra, S. Naya and R. Artiaga, Polym. Test., 2010, 29, 1002–1006 CrossRef PubMed.
- K. P. Menard, Dynamic Mechanical Analysis: A Practical Introduction, Taylor & Francis, 1999 Search PubMed.
- A. Zosel, Int. J. Adhes. Adhes., 1998, 18, 265–271 CrossRef CAS.
- Y. Li, D. Wang and X. Sun, J. Am. Oil Chem. Soc., 2015, 92, 121–131 CrossRef CAS PubMed.
- H. Yang and E. Chang, Trends Polym. Sci., 1997, 5, 380–384 CAS.
- C. A. Dahlquist, Tack, University of Nottingham, England, 1966 Search PubMed.
- E. P. Chang, J. Adhes., 1997, 60, 233–248 CrossRef CAS PubMed.
- E. P. Chang, J. Adhes., 1991, 34, 189–200 CrossRef CAS PubMed.
- M. Sherriff, R. W. Knibbs and P. G. Langley, J. Appl. Polym. Sci., 1973, 17, 3423–3438 CrossRef CAS PubMed.
- B. Yuan, C. McGlinchey and E. M. Pearce, J. Appl. Polym. Sci., 2006, 99, 2408–2413 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04399a |
| This journal is © The Royal Society of Chemistry 2015 |