Fabrication of intelligent poly(N-isopropylacrylamide)/silver nanoparticle composite films with dynamic surface-enhanced Raman scattering effect†
Abstract
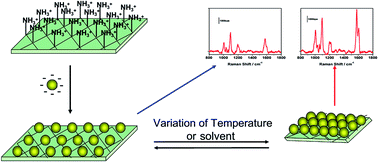
An intelligent PNIPAAm/AgNP composite film was fabricated by the simple assembly of silver nanoparticles on the surface of photo-polymerized PNIPAAm film via electrostatic interaction. Surface enhanced Raman scattering study and finite-difference time-domain simulation demonstrated that the local electric field intensity and the SERS intensity of this resultant composite film can be easily tuned by changing the interparticle distance triggered by temperature or solvent variations. The composite film showed a high stability in a polar organic solvent and concentrated saline solution. The smart composite film might find applications as a SERS sensor for temperature/solvent variation detection as well as a versatile SERS substrate for the detection of analyte in sea water and organic solvent.
- This article is part of the themed collection: Surface enhanced Raman Spectroscopy: Editors collection for RSC Advances


 Please wait while we load your content...
Please wait while we load your content...