High oxygen storage capacity and enhanced catalytic performance of NiO/NixCe1−xO2−δ nanorods: synergy between Ni-doping and 1D morphology
Abstract
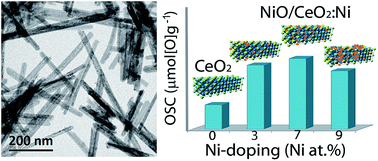
A series of Ni-doped ceria nanorods with low Ni content was synthesized via a hydrothermal approach and characterized by DRX, SEM, HRTEM, Raman and TPR techniques. Simultaneous tuning of morphology and composition, i.e. exposure of reactive (100) and (110) facets together with Ni substitution, provide great improvements in their physical chemical properties. Segregated NiO as a secondary phase was observed as Ni content increases affecting the growth of the nanorods. The system is described as NiO dispersed on NixCe1−xO2−δ nanorods. The materials showed a remarkably high OSC at 400 °C and good activity for CO oxidation. Results corroborate the synergic effects between Ni-doping and one-dimensional morphology on ceria structure.

 Please wait while we load your content...
Please wait while we load your content...