Efficient removal of atrazine in water with a Fe3O4/MWCNTs nanocomposite as a heterogeneous Fenton-like catalyst
Abstract
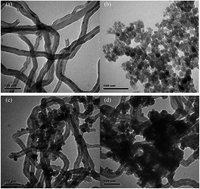
Fe3O4 and multi-walled carbon nanotube hybrid materials (Fe3O4/MWCNTs) were synthesized by a coprecipitation combined hydrothermal method. The nanocomposites were applied for adsorption and degradation of atrazine (ATZ) in the presence of H2O2. The obtained catalysts were characterized by TEM, XRD, BET, XPS and Raman spectroscopy. The effects of solution pH, catalysts dosage, H2O2 concentration and iron leaching on the degradation of ATZ were investigated. Fe3O4/MWCNTs showed a higher utilization efficiency of H2O2, higher ability of adsorption for ATZ and higher degradation efficiency of ATZ than Fe3O4 nanoparticles in the batch degradation experiment. The degradation efficiency increased with the solution pH decreasing from 8.0 to 3.0. The catalytic results showed that Fe3O4/MWCNTs presented good performance for the degradation of ATZ, achieving 81.4% decomposition of ATZ after 120 min at reaction conditions of H2O2 concentration 3.0 mmol L−1, catalysts dosage 0.1 g L−1, ATZ concentration 10.0 mg L−1, pH 5.0 and T 30 °C. Three degradation products (desethylatrazine, desisopropylatrazine, and 2-hydroxyatrazine) were detected during a heterogeneous Fenton reaction in solution. The stability, and reusability of Fe3O4/MWCNTs for ATZ degradation were also investigated.

 Please wait while we load your content...
Please wait while we load your content...