Preparation of functionalized castor oil derivatives with tunable physical properties using heterogeneous acid and base catalysts†
Abstract
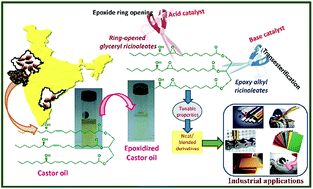
Functionalized castor oil derivatives namely ring-opened glyceryl ricinoleates, epoxy alkyl ricinoleates, and ring-opened alkyl ricinoleates were successfully prepared through two reaction chemistry viz., ring opening and transesterification using epoxidized castor oil (ECO) as a raw material. Amberlyst 15, the most active catalyst among several acid catalysts screened, showed a maximum conversion of 82% for ring opening of ECO with methanol. In another chemistry, 91% yield of epoxy methyl ricinoleate was achieved through transesterification of ECO with methanol using CaAl-layered double hydroxide (LDH) derived oxides as base catalyst. The scope is extendable to many nucleophiles and alcohols for both reactions respectively. Ring-opened alkyl ricinoleates were prepared both in two-pot and one-pot reactions using both acid and base catalysts together. The catalysts were recyclable and were successfully scaled at 25 g. The physical properties of these castor-based derivatives bestow the opportunity to design tailor-made materials suiting industrial needs.

 Please wait while we load your content...
Please wait while we load your content...