Low-temperature fabrication of high performance indium oxide thin film transistors
Abstract
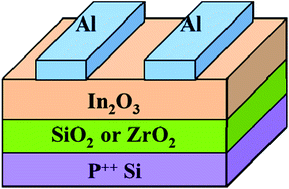
In this study, indium oxide (In2O3) thin-film transistors (TFTs) were fabricated by a solution-process at low temperature. A single precursor in a single solvent system was used as the In2O3 precursor to minimize the carbon-based impurities. The 300 °C-annealed In2O3 TFT with channel thickness of 12 nm exhibits enhanced performance, which shows saturation mobility (μsat) of 3.08 cm2 V−1 s−1, an on/off current ratio (Ion/Ioff) of 1.04 × 108, a threshold voltage (VT) of 12.7 V, and a subthreshold swing (SS) of 1.49 V per decade. Finally, high-performance In2O3 TFT based on solution-processed zirconium oxide dielectric was realized, which shows distinguished electrical performance (μsat = 13.01 cm2 V−1 s−1, Ion/Ioff = 1.09 × 107, VT = 1.2 V, and SS = 0.1 V per decade). These results suggest that solution-processed In2O3 TFTs could potentially be used for low-cost, low-temperature, and high-performance electronic devices.

 Please wait while we load your content...
Please wait while we load your content...