One-pot green synthesis of Ag/AgCl nanocube/reduced graphene oxide and its application to the simultaneous determination of hydroquinone and catechol
Abstract
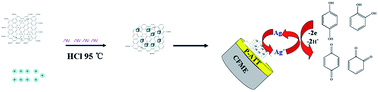
Uniformly dispersed Ag/AgCl nanocubes (AgNC) were successfully obtained on reduced graphene oxide (rGO) through the simultaneous reduction of Ag+ and graphene oxide (GO) by chitosan in the presence of a little HCl. The AgCl acted as a seed. The obtained nanocomposite was characterized by X-ray diffraction (XRD), energy dispersive X-ray spectroscopy (EDS) and scanning electron microscopy (SEM). The as-synthesized AgNC/rGO was immobilized on the surface of a poly(5-amino-1,3,4-thiadiazole-2-thiol) (p-ATT) modified carbon fiber disk ultramicroelectrode (CFME) for the simultaneous determination of hydroquinone (HQ) and catechol (CT). This sensor showed a wide linear range of 0.08–1000 μmol L−1 for HQ and 1.5–900 μmol L−1 for CT in the presence of 30 μmol L−1 of the counterpart.

 Please wait while we load your content...
Please wait while we load your content...