Synthesis of CdTe/P(St-nBA-AA) pierced fluorescent microspheres
Abstract
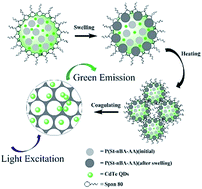
CdTe/P(St-nBA-AA) pierced fluorescent microspheres with a novel hollow morphology were prepared via a rapid inverse emulsion method. CdTe quantum dots (QDs), which were synthesized by a simple one-pot reflux process using thioglycolic acid as a stabilizer in an aqueous phase, were embedded into the polymer skeleton. The P(St-nBA-AA) component with a low glass transition temperature (Tg = 65 °C) was prepared by soap-free emulsion polymerization. Subsequently, the morphology, composition and spectral characteristics of the CdTe QDs and CdTe/P(St-nBA-AA) pierced fluorescent microspheres were determined by SEM, TEM, FTIR, TGA, XRD, laser particle analysis, and fluorescence spectrometry. The results indicated that the fluorescent microspheres had a relatively smooth surface and hollow internal structure. The QD content of the pierced fluorescent microspheres was 12.68%. Moreover, a synergistic effect mechanism of solvent swelling and phase transition was proposed. The formation process of pierced fluorescent microspheres was the swelling, movement, winding of the polymer chains of P(St-nBA-AA) particles, which were stabilized on the interface of the water/oil phase at temperatures higher than the Tg of the polymer component.

 Please wait while we load your content...
Please wait while we load your content...