PtAu alloy nanoparticles supported on thermally expanded graphene oxide as a catalyst for the selective oxidation of glycerol†
Gao-Yuan Yang,
Shuai Shao,
Yi-Hu Ke,
Chun-Ling Liu*,
Hui-Fang Ren and
Wen-Sheng Dong*
Key Laboratory of Applied Surface and Colloid Chemistry (SNNU), MOE, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an, 710062, China. E-mail: clliutt@snnu.edu.cn; wsdong@snnu.edu.cn; Fax: +86-29-81530806; Tel: +86-29-81530806
First published on 17th April 2015
Abstract
Thermally expanded graphene oxide (TEGO) supported PtAu alloy nanoparticles with various compositions was prepared, and then characterized using a combination of atomic absorption spectroscopy, powder X-ray diffraction, transmission electron microscopy and X-ray photoelectron spectroscopy. These catalysts were evaluated for the aerobic oxidation of glycerol in base-free aqueous solution. The results showed that PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO exhibited the optimum activity for the conversion of glycerol among all the catalysts. Glycerol conversion of 60.4% and selectivities of 53.5% glyceric acid (GLYA), 25.7% glyceraldehydes (GLYDE), 11.6% dihydroxyacetone (DHA), and 3.8% glycolic acid (GLYCA) were obtained when reacting an aqueous solution of glycerol (0.3 M, 20 mL) at 60 °C under 0.3 MPa O2 for 4 h in the presence of 0.023 g PtAu(7
1)/TEGO exhibited the optimum activity for the conversion of glycerol among all the catalysts. Glycerol conversion of 60.4% and selectivities of 53.5% glyceric acid (GLYA), 25.7% glyceraldehydes (GLYDE), 11.6% dihydroxyacetone (DHA), and 3.8% glycolic acid (GLYCA) were obtained when reacting an aqueous solution of glycerol (0.3 M, 20 mL) at 60 °C under 0.3 MPa O2 for 4 h in the presence of 0.023 g PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO catalyst. Moreover, the reusability of PtAu(7
1)/TEGO catalyst. Moreover, the reusability of PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO was investigated, and a reaction mechanism for the oxidation of glycerol was proposed.
1)/TEGO was investigated, and a reaction mechanism for the oxidation of glycerol was proposed.
1. Introduction
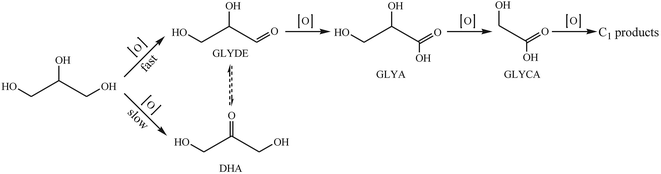
During the past decade, the catalytic conversion of biomass and derivatives to chemicals has attracted extensive concern.1 The oxidation of sugars, glycerol, and HMF for the production of chemicals is an important research topic in the transformation of biomass. The application of supported metal particles and molecular oxygen for the oxidation of oxygenated compounds offers a green alternative to classical chemical oxidants.2Glycerol is currently produced in large amounts as a byproduct of the manufacture of biodiesel by the transesterification of vegetable oils and animal fats. The conversion of the byproduct glycerol into value-added products would make the biodiesel industry economically more attractive. The oxidation of glycerol could follow complex reaction pathways yielding various C3 oxygenates (glyceric acid, glyceraldehyde, dihydroxyacetone, tartronic acid, etc.) together with C2 (oxalic and glycolic acids) and C1 (formic acid) products. Hence, it is still a challenge to control the selectivity to give desired products by choosing highly efficient catalysts.2
The aerobic oxidation of glycerol has been extensively studied using monometallic metal Pt,3–9 Pd,10,11 Au12–18 et al. A major disadvantage of supported Pt-group catalysts is their rapid deactivation by overoxidation of the metal surface and by poisoning of the active sites by strongly adsorbed products or byproducts.8,19 Bimetallic catalysts lead to a strong synergistic effect and show better catalytic performance with respect to monometallic systems. So far, various bi-metal catalysts have been examined in the aerobic oxidation of glycerol in base-free aqueous solution.20–25 For example, Villa et al.26 found only 5% conversion of glycerol and 70% selectivity to glyceric acid over a monometallic 1% Au/H-mordenite catalyst at 100 °C for 2 h under 3 atm O2, but 70% conversion of glycerol and 83% selectivity to glyceric acid over a bimetallic 1% (Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt 6
Pt 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)/H-mordenite. Hutchings et al.27 observed 29.2% glycerol conversion and 78.4% glyceric acid selectivity over AuPt(1
4)/H-mordenite. Hutchings et al.27 observed 29.2% glycerol conversion and 78.4% glyceric acid selectivity over AuPt(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/MgO after reaction at 60 °C for 4 h under 0.3 MPa O2. While 42.9% conversion of glycerol and 78.4% selectivity to glyceric acid were obtained over AuPt(1
1)/MgO after reaction at 60 °C for 4 h under 0.3 MPa O2. While 42.9% conversion of glycerol and 78.4% selectivity to glyceric acid were obtained over AuPt(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/MgO. More recently, Tongsakul et al.28 investigated the aerobic oxidation of glycerol using hydrotalcite (HT)-supported Au–Pt alloy nanoparticles, and found that Pt60Au40/HT was the most active catalyst in the reaction, giving 73% conversion of glycerol and 78% selectivity to glyceric acid at ambient temperature for 4 h under oxygen flow. Although bimetallic catalysts have been found exhibiting better catalytic performance than monometallic systems, the influence of the composition and internal structure of the alloy, and the understanding of the mechanism by which they give enhanced performances, still remains an open field of research.
3)/MgO. More recently, Tongsakul et al.28 investigated the aerobic oxidation of glycerol using hydrotalcite (HT)-supported Au–Pt alloy nanoparticles, and found that Pt60Au40/HT was the most active catalyst in the reaction, giving 73% conversion of glycerol and 78% selectivity to glyceric acid at ambient temperature for 4 h under oxygen flow. Although bimetallic catalysts have been found exhibiting better catalytic performance than monometallic systems, the influence of the composition and internal structure of the alloy, and the understanding of the mechanism by which they give enhanced performances, still remains an open field of research.
Recently, graphene, a one-atom-thick planar sheet of carbon that is densely packed in a honeycomb crystal lattice, has attracted extensive attention as a catalyst support because of its high surface area and outstanding electrical conductivity.29–32 Both surfaces of the sheet are accessible for reactants, which would reduce the diffusion limitations and promoted the reaction efficiency.30,32 Truong-Huu et al.30 have found that palladium nanoparticles (NPs) dispersed on a few-layer graphene exhibited high activity for cinnamaldehyde hydrogenation than those supported on the 1D carbon nanotubes.
Inspired by the excellent performance of the graphene supported catalysts in the various reactions, we prepared bi-metallic platinum–gold nanoparticles dispersed on thermal expanded graphene oxide and used these materials to catalyze the oxidation of glycerol in base-free aqueous solution. This novel catalyst shows good catalytic performance for the efficient conversion of glycerol.
2. Experimental
2.1 Chemicals
Polyvinyl alcohol (PVA, Mw = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 80% hydrolyzed) and glycolic acid (GLYCA) were purchased from Sigma-Aldrich. H2PtCl6·6H2O, HAuCl4, NaBH4, NaNO3, KMnO4, 30% H2O2, graphite powder, and sulfuric acid were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Glycerol was purchased from Fuchen Chemical Reagent (Tianjin, China). Glyceric acid (GLYA, 40% in water), glyceraldehyde (GLYDE) were obtained from Alfa Aesar. Dihydroxyacetone (DHA) was obtained from J&K CHEMICA.
000, 80% hydrolyzed) and glycolic acid (GLYCA) were purchased from Sigma-Aldrich. H2PtCl6·6H2O, HAuCl4, NaBH4, NaNO3, KMnO4, 30% H2O2, graphite powder, and sulfuric acid were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Glycerol was purchased from Fuchen Chemical Reagent (Tianjin, China). Glyceric acid (GLYA, 40% in water), glyceraldehyde (GLYDE) were obtained from Alfa Aesar. Dihydroxyacetone (DHA) was obtained from J&K CHEMICA.
All chemicals were of analytical grade and were used as received without further purification.
2.2 Preparation of catalysts
Graphite oxide was synthesized from natural graphite powder according to the following procedure. First, graphite (2.5 g), NaNO3 (1.0 g) and H2SO4 (46 mL) were mixed and stirred for 30 min in an ice bath, and then KMnO4 (4.5 g) was added. The mixture solution was stirred for 120 min at room temperature, and then water (130 mL) was added for dilution. The solution was further refluxed for 40 min. After the reaction, the residual KMnO4 was removed by adding 30% H2O2 (10 mL), the yellow precipitate was separated by centrifugation and washed with water repeatedly. Graphite oxide (GO) powder was obtained after drying the precipitate. Graphite oxide powder was expanded in a muffle furnace pre-heated to 1000 °C for 10 s in air, and then the thermally expanded graphene oxide (TEGO) nanosheets were obtained.Supported Pt–Au catalysts were prepared by immobilizing the colloidal metal particles on the TEGO supports. In a typical preparation, the protecting agent was added (PVA/metal mass ratio = 0.5) to a 50 mL aqueous solution of H2PtCl6·6H2O and HAuCl4 (1.12 × 10−3 M) at room temperature (25 °C) under vigorous stirring. A following rapid injection of a 0.1 M aqueous solution of NaBH4 (NaBH4/metal molar ratio = 4) led to the formation of a dark solution, indicating the formation of metallic colloids. The pH of the colloidal solution needed to be adjusted to 2 with sulfuric acid prior to the immobilization. The immobilization of metallic particles on the TEGO material was accomplished at room temperature (25 °C) by adding the support to the colloidal solution by ultrasonication and they were kept in contact until total adsorption (10 wt% metal on the support) occurred, as indicated by a decoloration of the solution. The solids were collected by filtration followed by washing the solid with distilled water to remove all the dissolved species (e.g. Na+, Cl−). Finally, the solids were dried under vacuum at 110 °C for 12 h.
2.3 Catalyst characterization
X-ray diffraction (XRD) patterns of the catalysts were collected on a Bruker D8 Advance X-ray diffractometer using Cu Kα radiation (40 kV, 30 mA). Transmission electron microscopy (TEM) was conducted on a JEOL JEM 2010 electron microscope operating at 200 kV. X-ray photoelectron spectroscopy (XPS) analysis was performed on an Axis Ultra Kratos (UK) apparatus using Al Kα source (15 kV, 1486.6 eV). The binding energy was calibrated relative to the C1s peak (284.8 eV) of the contaminant carbon. Pt and Au contents in the catalysts were obtained with a Hitachi ZA3000 atomic absorption spectrometer (AAS).2.4 Reaction test and product analysis
All the reactions were carried out in a 35 mL stainless steel autoclave equipped with a mechanical stirrer. In a typical experiment, 0.023 g catalyst powder and 20 mL aqueous solution of glycerol (0.3 M) were charged into the reactor. The autoclave was purged two times with pure O2 and then pressurized to 0.3 MPa with O2 at room temperature. The reaction mixture was heated to 60 °C and held at the temperature for 4 h unless otherwise stated under a stirring rate of 600 rpm. After the reaction, the reactor was cooled quickly down to room temperature with an ice–water mixture and depressurized. The post-reaction liquid sample was diluted with mobile phase solution before analysis.Sample analysis was performed on a Shimadzu HPLC LC-20AT system equipped with both refractive index (RID-10A) and UV (SPD-20A) detectors. An ion exclusion column (Bio-Rad Aminex HPX-87H) at 40 °C was used to separate and identify the compounds. In order to well separate the products, two mobile phases flowing at 0.30 mL min−1 were used. One was aqueous 0.005 M H2SO4, and the other was 0.005 M HCOOH. The amount of products was determined by using calibration curves.
3. Results and discussion
3.1 Structure characterization of PtAu/TEGO catalysts
The compositions of the as-prepared catalysts were analyzed by AAS, and the results are summarized in Table 1. The total metal contents are near 10% in all the catalysts, indicating that Pt and Au nanoparticles were well deposited on the surface of the TEGO.| Catalyst | Au (wt%) | Pt (wt%) | Total metal loading (wt%) | Pt/Au atomic ratio |
|---|---|---|---|---|
| Pt/TEGO | — | 9.8 | 9.8 | — |
PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO 1)/TEGO |
1.3 | 8.9 | 10.2 | 6.9 |
PtAu(5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO 1)/TEGO |
1.6 | 8.5 | 10.1 | 5.4 |
PtAu(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO 1)/TEGO |
2.3 | 7.6 | 9.9 | 3.3 |
PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO 1)/TEGO |
4.9 | 5.4 | 10.3 | 1.1 |
PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/TEGO 3)/TEGO |
8.0 | 2.0 | 10.0 | 0.25 |
| Au/TEGO | 9.6 | — | 9.6 | — |
PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO-used 1)/TEGO-used |
0.5 | 8.6 | 9.1 | 17.4 |
The XRD patterns of Pt/TEGO, Au/TEGO, and PtAu/TEGO catalysts are shown in Fig. 1. A broad peak centered at ∼24° was observed for all the samples, which was ascribed to the formation of “re-graphitized” carbon regions and restacking due to the van der Waals attractive interactions.31 No diffraction peaks at 10.8° from graphite oxide were observed, indicating that the graphite oxide was partially reduced by the thermal expansion at high temperature, without excessive covalent chemical functionalization as in the case of GO.32 The XRD pattern of Pt/TEGO showed a strong reflection peak at 39.7° and a weak peak at 46.4°, corresponding to (1 1 1) and (2 0 0) crystal planes of Pt, respectively. For Au/TEGO, a strong peak at 38.3° and a weak peak at 44.6° were observed, which could be assigned to (1 1 1) and (2 0 0) crystal planes of Au, respectively. All the XRD patterns of PtAu/TEGO revealed the face-centered cubic structure of Pt–Au alloy. The diffraction peak of (1 1 1) plane gradually shifted from 2θ = 38.3°, 38.5°, 38.6°, 38.8°, 38.9°, 39.1°, and 39.7° with increasing the content of Pt in the PtAu/TEGO catalysts, confirming the formation of Pt–Au alloy in the PtAu/TEGO catalysts.28
 | ||
Fig. 1 XRD patterns for various graphene supported catalysts: (a) Pt/TEGO, (b) PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, (c) PtAu(5 1)/TEGO, (c) PtAu(5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, (d) PtAu(3 1)/TEGO, (d) PtAu(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, (e) PtAu(1 1)/TEGO, (e) PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, (f) PtAu(1 1)/TEGO, (f) PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/TEGO, (g) Au/TEGO. 3)/TEGO, (g) Au/TEGO. | ||
TEM micrographs and mean particle size distribution histograms for all catalysts are shown in Fig. 2. The corresponding HRTEM images of the bimetallic catalysts are shown in Fig. s1.† Bimetallic nanoparticles were well dispersed on the surface of TEGO and showed a narrow size distribution. The average Pt particle size in Pt/TEGO was 2.2 nm; the average Au particle size in Au/TEGO was 2.8 nm. Increasing the amounts of Au in the PtAu/TEGO catalysts had a slight effect on the mean particle sizes of Pt–Au alloy. The HRTEM images showed that lattice fringes of Pt–Au alloy particles were 0.225, 0.227, 0.229, 0.229 and 0.231 nm for PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, PtAu(5
1)/TEGO, PtAu(5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, PtAu(3
1)/TEGO, PtAu(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, PtAu(1
1)/TEGO, PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO and PtAu(1
1)/TEGO and PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/TEGO, respectively, which were different from the (1 1 1) lattice spacings Au (0.235 nm) and Pt (0.226 nm), further confirming the formation of Pt–Au alloy.
3)/TEGO, respectively, which were different from the (1 1 1) lattice spacings Au (0.235 nm) and Pt (0.226 nm), further confirming the formation of Pt–Au alloy.
XPS was used to analyze the surface element composition and content of the catalysts, and the results are summarized in Table 2. The representative spectra for PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO are shown in Fig. s2.† From the C1s spectrum three different peaks centered at 284.8, 286.3, and 288.8 eV were observed, corresponding to C–C, C–O, and C
1)/TEGO are shown in Fig. s2.† From the C1s spectrum three different peaks centered at 284.8, 286.3, and 288.8 eV were observed, corresponding to C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively. The C–C group occupied the vast majority, revealing that the oxygen-containing functional groups were mostly removed and the conjugated graphene networks were kept after the thermal expansion at high temperature.33 In addition, XPS measurements revealed that the C/O ratio (5.1–6.1) of the TEGO supported catalyst was evidently more than that of graphene oxide (1.7–2.5), and less than that of graphene (10.8–14.9), further confirming that the graphite oxide was partially reduced by the thermal expansion at high temperature.32 The valence state information of Au and Pt was also studied by XPS and the results are shown in Table 2 and Fig. s1.† The binding energy of Au4f7/2 in each sample was almost constant (∼84.2 eV), indicating that Au was present as metallic state and no electronic interaction between Au–TEGO and Au–Pt. The binding energy of Pt4f7/2 in each sample was ∼71.7 eV except for PtAu(1
O groups, respectively. The C–C group occupied the vast majority, revealing that the oxygen-containing functional groups were mostly removed and the conjugated graphene networks were kept after the thermal expansion at high temperature.33 In addition, XPS measurements revealed that the C/O ratio (5.1–6.1) of the TEGO supported catalyst was evidently more than that of graphene oxide (1.7–2.5), and less than that of graphene (10.8–14.9), further confirming that the graphite oxide was partially reduced by the thermal expansion at high temperature.32 The valence state information of Au and Pt was also studied by XPS and the results are shown in Table 2 and Fig. s1.† The binding energy of Au4f7/2 in each sample was almost constant (∼84.2 eV), indicating that Au was present as metallic state and no electronic interaction between Au–TEGO and Au–Pt. The binding energy of Pt4f7/2 in each sample was ∼71.7 eV except for PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/TEGO, indicating that a small positive shift to high binding energy as compared with Pt foil (Pt 4f7/2: 71.2 eV). The results suggest that Pt was present as Ptδ+ because the surface of Pt0 could be easily oxidized when exposed to air. For PtAu(1
3)/TEGO, indicating that a small positive shift to high binding energy as compared with Pt foil (Pt 4f7/2: 71.2 eV). The results suggest that Pt was present as Ptδ+ because the surface of Pt0 could be easily oxidized when exposed to air. For PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/TEGO, the binding energy of Pt4f7/2 was 71.1 eV, suggesting that Pt species in this catalyst was mainly present in metallic state because this catalyst contained more Au that might prevented the oxidation of Pt. As shown in Tables 1 and 2, the surface contents of Pt and Au were higher than those in the bulk of the catalysts, indicating the enrichment of Pt and Au on the surface. Moreover, the surface Pt/Au atomic ratios for PtAu(3
3)/TEGO, the binding energy of Pt4f7/2 was 71.1 eV, suggesting that Pt species in this catalyst was mainly present in metallic state because this catalyst contained more Au that might prevented the oxidation of Pt. As shown in Tables 1 and 2, the surface contents of Pt and Au were higher than those in the bulk of the catalysts, indicating the enrichment of Pt and Au on the surface. Moreover, the surface Pt/Au atomic ratios for PtAu(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, PtAu(5
1)/TEGO, PtAu(5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, and PtAu(7
1)/TEGO, and PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO were 2.3, 2.7, and 3.3, respectively; the bulk Pt/Au ratios for the corresponding catalysts were 3.3, 5.4, and 6.9, respectively. The results suggest that Au was relatively enriched on the surface of Au–Pt alloy in these three catalysts. Whereas, for PtAu(1
1)/TEGO were 2.3, 2.7, and 3.3, respectively; the bulk Pt/Au ratios for the corresponding catalysts were 3.3, 5.4, and 6.9, respectively. The results suggest that Au was relatively enriched on the surface of Au–Pt alloy in these three catalysts. Whereas, for PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO, PtAu(1
1)/TEGO, PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/TEGO the situations were reversed, Pt was relatively enriched on the surface of Au–Pt alloy in these two catalysts.
3)/TEGO the situations were reversed, Pt was relatively enriched on the surface of Au–Pt alloy in these two catalysts.
| Catalysts | Binding Energy (eV) | Surface concentration (wt%) | Pt/Au atomic ratio | ||||
|---|---|---|---|---|---|---|---|
| Au4f7/2 | Pt4f7/2 | C | O | Pt | Au | ||
| Pt/TEGO | — | 71.7 | 63.6 | 14.3 | 22.0 | 0.0 | — |
PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO 1)/TEGO |
84.2 | 71.8 | 60.5 | 13.9 | 19.6 | 6.0 | 3.3 |
PtAu(5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO 1)/TEGO |
84.2 | 71.7 | 59.4 | 14.2 | 19.3 | 7.1 | 2.7 |
PtAu(3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO 1)/TEGO |
84.2 | 71.7 | 58.4 | 13.7 | 18.8 | 8.1 | 2.3 |
PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO 1)/TEGO |
84.2 | 71.7 | 61.0 | 13.8 | 16.3 | 8.8 | 1.9 |
PtAu(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/TEGO 3)/TEGO |
84.2 | 71.1 | 63.1 | 13.7 | 13.4 | 9.8 | 1.4 |
| Au/TEGO | 84.3 | — | 67.2 | 17.5 | 0.0 | 15.3 | — |
PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO-used 1)/TEGO-used |
— | 71.9 | 62.5 | 14.8 | 22.7 | 0 | — |
3.2 Catalytic performance of Pt–Au/TEGO catalysts
Fig. 3 summarizes the activity of the PtAu/TEGO catalysts for the oxidation of glycerol in base-free aqueous solution. The main products on the catalysts were glyceric acid, glyceraldehyde together with small amounts of glycolic acid, dihydroxyacetone, and C1 products. Au/TEGO showed almost no activity for the aerobic oxidation of glycerol in base-free aqueous solution. The result was in accordance with the data reported by Hutchings et al.11 Over Pt/TEGO, the conversion of glycerol was 50%, the selectivities of glyceric acid, glyceraldehydes, glycolic acid, and dihydroxyacetone 42.7%, 14.5%, 9.2%, and 12.1%, respectively. With increasing the Pt/Au ratio in the PtAu/TEGO catalysts from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 7
3 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the conversion of glycerol increased monotonously from 14.6% to 60.4%, indicating that increasing Pt content could promote the conversion of glycerol. The PtAu(7
1, the conversion of glycerol increased monotonously from 14.6% to 60.4%, indicating that increasing Pt content could promote the conversion of glycerol. The PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO revealed a higher conversion of glycerol (60.4%) than Pt/TEGO (50%), suggesting that the presence of a synergistic effect between Pt and Au. With increasing the Pt/Au ratio in the PtAu/TEGO catalysts from 1
1)/TEGO revealed a higher conversion of glycerol (60.4%) than Pt/TEGO (50%), suggesting that the presence of a synergistic effect between Pt and Au. With increasing the Pt/Au ratio in the PtAu/TEGO catalysts from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 7
3 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the selectivity of glyceric acid decreased roughly; whereas the selectivities of glycolic acid and dihydroxyacetone increased gradually. The results suggest that the addition of Au could enhance the selectivity of glyceric acid, but decreased the selectivities of glycolic acid and dihydroxyacetone because the addition of Au to Pt can inhibit C–C scission.25 Among all of the catalysts, PtAu(7
1, the selectivity of glyceric acid decreased roughly; whereas the selectivities of glycolic acid and dihydroxyacetone increased gradually. The results suggest that the addition of Au could enhance the selectivity of glyceric acid, but decreased the selectivities of glycolic acid and dihydroxyacetone because the addition of Au to Pt can inhibit C–C scission.25 Among all of the catalysts, PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO showed the best activity for the conversion of glycerol, hence this catalyst was selected for further studies.
1)/TEGO showed the best activity for the conversion of glycerol, hence this catalyst was selected for further studies.
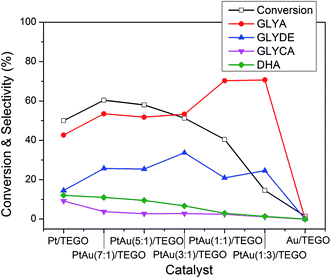 | ||
| Fig. 3 Oxidation of glycerol using various TEGO supported catalysts in water (catalyst 0.023 g, glycerol 6 mmol, water 20 mL, glycerol/metal = 750 (mol mol−1), 60 °C, 0.3 MPa O2). | ||
Fig. 4 shows the time course of glycerol oxidation on PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO in base-free aqueous solution. At the initial stage of the oxidation reaction (1.0 h), glycerol conversion was 21.8%. The corresponding selectivities of glyceric acid, glyceraldehyde, glycolic acid, and dihydroxyacetone were 69.5%, 10.7%, 4.8%, and 6.4%, respectively. With prolonged reaction time, glycerol conversion increased and the selectivity of glyceric acid gradually decreased, whereas the selectivities of glyceraldehyde and dihydroxyacetone roughly increased, indicating that as the reaction was in progress the oxidation rate of glyceraldehyde to glyceric acid decreased.
1)/TEGO in base-free aqueous solution. At the initial stage of the oxidation reaction (1.0 h), glycerol conversion was 21.8%. The corresponding selectivities of glyceric acid, glyceraldehyde, glycolic acid, and dihydroxyacetone were 69.5%, 10.7%, 4.8%, and 6.4%, respectively. With prolonged reaction time, glycerol conversion increased and the selectivity of glyceric acid gradually decreased, whereas the selectivities of glyceraldehyde and dihydroxyacetone roughly increased, indicating that as the reaction was in progress the oxidation rate of glyceraldehyde to glyceric acid decreased.
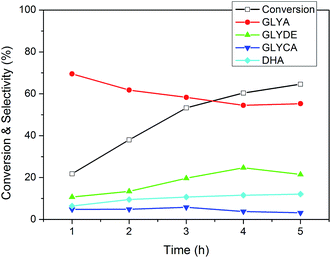 | ||
Fig. 4 Time course of glycerol oxidation over PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO in water (catalyst 0.023 g, glycerol 6 mmol, water 20 mL, glycerol/metal = 750 (mol mol−1), 60 °C, 0.3 MPa O2). 1)/TEGO in water (catalyst 0.023 g, glycerol 6 mmol, water 20 mL, glycerol/metal = 750 (mol mol−1), 60 °C, 0.3 MPa O2). | ||
The oxidation of glycerol could follow complex reaction pathways yielding various oxygenated products.2,19 The oxidation of a primary or secondary alcohol produces glyceraldehyde or dihydroxyacetone, respectively. These two produces are often in equilibrium in aqueous solution, depending on the pH.19 Both glyceric acid and dihydroxyacetone can be oxidized to hydroxypyruvic acid. Tartronic acid is produced by the sequential oxidation of glyceric acid, while glycolic acid is the product of sequential oxidation of glyceric acid and tartronic acid. The sequential oxidation of glycolic acid and hydroxypyruvic acid generates oxalic acid and one carbon products (formic acid, COx). In the present study, the main products were glyceric acid and glyceraldehyde together with small amounts of dihydroxyacetone, glycolic acid, and trace amounts of C1 products, suggesting that the formation rate of dihydroxyacetone over PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO was lower than that of glyceraldehyde. Therefore, a plausible reaction pathway for the oxidation of glycerol over PtAu(7
1)/TEGO was lower than that of glyceraldehyde. Therefore, a plausible reaction pathway for the oxidation of glycerol over PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO in base-free aqueous solution is proposed in Scheme 1.
1)/TEGO in base-free aqueous solution is proposed in Scheme 1.
The reusability of PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO in the oxidation of glycerol was investigated. After each trial, the catalyst was separated from the reaction solution by filtration, washed with distilled water, dried and then used for the next run under identical reaction conditions. The results in Fig. 5 revealed that both the conversion of glycerol and the selectivity of glyceric acid decreased; whereas the selectivites of glyceraldehyde and dihydroxyacetone increased gradually.
1)/TEGO in the oxidation of glycerol was investigated. After each trial, the catalyst was separated from the reaction solution by filtration, washed with distilled water, dried and then used for the next run under identical reaction conditions. The results in Fig. 5 revealed that both the conversion of glycerol and the selectivity of glyceric acid decreased; whereas the selectivites of glyceraldehyde and dihydroxyacetone increased gradually.
 | ||
Fig. 5 Recycling of PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO during the oxidation of glycerol (catalyst 0.023 g, glycerol 6 mmol, water 20 mL, glycerol/metal = 750 (mol mol−1), 60 °C, 0.3 MPa O2, 4 h). 1)/TEGO during the oxidation of glycerol (catalyst 0.023 g, glycerol 6 mmol, water 20 mL, glycerol/metal = 750 (mol mol−1), 60 °C, 0.3 MPa O2, 4 h). | ||
To determine the real reasons underlying catalyst deactivation, the used PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO catalyst was characterized by XRD, TEM, XPS, and AAS analysis. AAS analysis (Table 1) indicated that contents of Au and Pt in PtAu(7
1)/TEGO catalyst was characterized by XRD, TEM, XPS, and AAS analysis. AAS analysis (Table 1) indicated that contents of Au and Pt in PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO decreased from 1.3% to 0.5%, and 8.9% to 8.6% after four recycling, respectively. XPS analysis (Fig. s1,† and Table 2) showed that no Au signal was detected on the used catalyst surface; while Pt content increased from 19.6% to 22.7%. The results indicated that the leaching of Au could occur after recycling. Moreover, XRD pattern (Fig. s3†) confirmed that the (1 1 1) diffraction peak of Pt–Au alloy increased in intensity. TEM image (Fig. s4†) showed that the small alloy particles agglomerated together to form larger clusters after recycling. Hence, the decrease of catalytic activity of PtAu(7
1)/TEGO decreased from 1.3% to 0.5%, and 8.9% to 8.6% after four recycling, respectively. XPS analysis (Fig. s1,† and Table 2) showed that no Au signal was detected on the used catalyst surface; while Pt content increased from 19.6% to 22.7%. The results indicated that the leaching of Au could occur after recycling. Moreover, XRD pattern (Fig. s3†) confirmed that the (1 1 1) diffraction peak of Pt–Au alloy increased in intensity. TEM image (Fig. s4†) showed that the small alloy particles agglomerated together to form larger clusters after recycling. Hence, the decrease of catalytic activity of PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO during recycling could be due to the combination of the agglomeration of Pt particles, and the leaching of Au.
1)/TEGO during recycling could be due to the combination of the agglomeration of Pt particles, and the leaching of Au.
4. Conclusions
We prepared novel thermally expanded graphene oxide supported Pt–Au bimetallic catalysts. The Pt–Au nanoparticles with the average size of ∼2.6 nm were homogenously dispersed on the surface of the thermally expanded graphene oxide. These catalysts were used in the selective oxidation of glycerol with molecular oxygen in a base-free aqueous solution. The results showed that that with increasing the Pt/Au ratio in the PtAu/TEGO catalysts from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 7
3 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the conversion of glycerol increased monotonously from 14.6% to 60.4%; the selectivity of glyceric acid decreased roughly; whereas the selectivities of glycolic acid and dihydroxyacetone increased gradually. Among all of the catalysts, PtAu(7
1, the conversion of glycerol increased monotonously from 14.6% to 60.4%; the selectivity of glyceric acid decreased roughly; whereas the selectivities of glycolic acid and dihydroxyacetone increased gradually. Among all of the catalysts, PtAu(7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/TEGO showed the best activity for the conversion of glycerol. Upon recycling of the catalyst, the conversion of glycerol decreased gradually. The decrease of catalytic activity during recycling could be due to the combination of agglomeration of Pt particles, and the leaching of Au.
1)/TEGO showed the best activity for the conversion of glycerol. Upon recycling of the catalyst, the conversion of glycerol decreased gradually. The decrease of catalytic activity during recycling could be due to the combination of agglomeration of Pt particles, and the leaching of Au.
Acknowledgements
The authors gratefully acknowledge financial support from the Program for Key Science and Technology Innovation Team of Shaanxi Province (2012KCT-21), the Program for Changjiang Scholars and Innovative Research Team in University of China (IRT_14R33), and the Fundamental Research Funds for the Central Universities (GK201501007).References
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 CrossRef CAS PubMed.
- A. Tsuji, K. T. V. Rao, S. Nishimura, A. Takagaki and K. Ebitani, ChemSusChem, 2011, 4, 542–548 CrossRef CAS PubMed.
- E. G. Rodrigues, M. F. R. Pereira, X. Chen, J. J. Delgado and J. J. M. Órfäo, Ind. Eng. Chem. Res., 2013, 52, 17390–17398 CrossRef CAS.
- D. Liang, J. Gao, J. Wang, P. Chen, Z. Hou and X. Zheng, Catal. Commun., 2009, 10, 1586–1590 CrossRef CAS PubMed.
- J. Gao, D. Liang, P. Chen, Z. Hou and X. Zheng, Catal. Lett., 2009, 130, 185–191 CrossRef CAS.
- D. Liang, J. Gao, H. Sun, P. Chen, Z. Hou and X. Zheng, Appl. Catal., B, 2011, 106, 423–432 CrossRef CAS PubMed.
- F. F. Wang, S. Shao, C. L. Liu, C. L. Xu, R. Z. Yang and W. S. Dong, Chem. Eng. J., 2015, 264, 336–343 CrossRef CAS PubMed.
- C. Jin, C. Sun, R. Dong and Z. Chen, J. Nanosci. Nanotechnol., 2012, 12, 324–329 CrossRef CAS PubMed.
- M. Sankar, N. Dimitratos, D. W. Knight, A. F. Carley, R. Tiruvalam, C. J. Kiely, D. Thomas and G. J. Hutchings, ChemSusChem, 2009, 2, 1145–1151 CrossRef CAS PubMed.
- S. Carrettin, P. McMorn, P. Johnston, K. Griffin, C. J. Kiely and G. J. Hutchings, Phys. Chem. Chem. Phys., 2003, 5, 1329–1336 RSC.
- S. Carrettin, P. McMorn, P. Johnston, K. Griffin and G. J. Hutchings, Chem. Commun., 2002, 696–697 RSC.
- F. Porta and L. Prati, J. Catal., 2004, 224, 397–403 CrossRef CAS PubMed.
- S. Demirel, K. Lehnert, M. Lucas and P. Claus, Appl. Catal., B, 2007, 70, 637–643 CrossRef CAS PubMed.
- E. G. Rodrigues, S. A. C. Carabineiro, J. J. Delgado, X. Chen, M. F. R. Pereira and J. J. M. Orfao, J. Catal., 2012, 285, 83–91 CrossRef CAS PubMed.
- S. Gil, L. Munoz, L. Sanchez-Silva, A. Romero and J. L. Valverde, Chem. Eng. J., 2011, 172, 418–429 CrossRef CAS PubMed.
- S. Gil, M. Marchenab, C. M. Fernándezb, L. Sánchez-Silvab, A. Romeroa and J. L. Valverdeb, Appl. Catal., A, 2013, 450, 189–203 CrossRef CAS PubMed.
- S. S. Liu, K. Q. Sun and B. Q. Xu, ACS Catal., 2014, 4, 2226–2230 CrossRef CAS.
- S. E. Davis, M. S. Ide and R. J. Davis, Green Chem., 2013, 15, 17–45 RSC.
- W. C. Ketchie, M. Murayama and R. J. Davis, J. Catal., 2007, 250, 264–273 CrossRef CAS PubMed.
- N. Dimitratos, J. A. Lopez-Sanchez, J. M. Anthonykutty, G. Brett, A. F. Carley, R. C. Tiruvalam, A. A. Herzing, C. J. Kiely, D. W. Knight and G. J. Hutchings, Phys. Chem. Chem. Phys., 2009, 11, 4952–4961 RSC.
- S. Hirasawa, Y. Nakagawa and K. Tomishige, Catal. Sci. Technol., 2012, 2, 1150–1152 CAS.
- D. Liang, J. Gao, J. Wang, P. Chen, Y. Wei and Z. Hou, Catal. Commun., 2011, 12, 1059–1062 CrossRef CAS PubMed.
- R. Nie, D. Liang, L. Shen, J. Gao, P. Chen and Z. Hou, Appl. Catal., B, 2012, 127, 212–220 CrossRef CAS PubMed.
- S. A. Kondrat, P. J. Miedziak, M. Douthwaite, G. L. Brett, T. E. Davies, D. J. Morgan, J. K. Edwards, D. W. Knight, C. J. Kiely, S. H. Taylor and G. J. Hutchings, ChemSusChem, 2014, 7, 1326–1334 CrossRef CAS PubMed.
- A. Villa, G. M. Veith and L. Prati, Angew. Chem., Int. Ed., 2010, 49, 4499–4502 CrossRef CAS PubMed.
- G. L. Brett, Q. He, C. Hammond, P. J. Miedziak, N. Dimitratos, M. Sankar, A. A. Herzing, M. Conte, J. A. Lopez-Sanchez, C. J. Kiely, D. W. Knight, S. H. Taylor and G. J. Hutchings, Angew. Chem., Int. Ed., 2011, 50, 10136–10139 CrossRef CAS PubMed.
- D. Tongsakul, S. Nishimura and K. Ebitani, ACS Catal., 2013, 3, 2199–2207 CrossRef CAS.
- X. Jin, L. Dang, J. Lohrman, B. Subramaniam, S. Ren and R. V. Chaudhari, ACS Nano, 2013, 7, 1309–1316 CrossRef CAS PubMed.
- T. Truong-Huu, K. Chizaria, I. Janowskaa, M. S. Moldovanb, O. Ersenb, L. D. Nguyenc, M. J. Ledoux, C. Pham-Huua and D. Begin, Catal. Today, 2012, 189, 77–82 CrossRef CAS PubMed.
- R. Nie, J. Wang, L. Wang, Y. Qin, P. Chen and Z. Hou, Carbon, 2012, 50, 586–596 CrossRef CAS PubMed.
- Z. Rong, Z. Sun, Y. Wang, J. Lv and Y. Wang, Catal. Lett., 2014, 144, 980–986 CrossRef CAS PubMed.
- Z.-S. Wu, W. Ren, L. Gao, B. Liu, C. Jiang and H.-M. Cheng, Carbon, 2009, 47, 493–499 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04048e |
| This journal is © The Royal Society of Chemistry 2015 |