Synthesis, microstructure and properties of Ti–Al porous intermetallic compounds prepared by a thermal explosion reaction†
Abstract
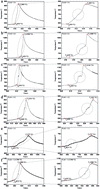
Ti–Al porous intermetallic compounds were prepared by a simple and energy-saving process of thermal explosion (TE) reactions. The effects of the Ti/Al molar ratios (including Ti : Al = 3 : 1, 2 : 1, 1 : 1, 1 : 2 and 1 : 3) on the temperature profiles, phase compositions, pore characteristics, density, expansion behaviors and oxidation resistance were investigated. The ignition temperatures were between 630–653 °C and the combustion temperatures were between 737–1161 °C, both of which are higher than the target furnace temperature. Porous Ti–Al alloys displayed a high open porosity of 35–56%. The pores were from non-fully dense green compacts and expansion behaviors of TE. When the target furnace temperature was set at 650 °C, the specimen with Ti and Al in a 1 : 2 ratio possessed the highest open porosity of 53.89% and the lowest density of 1.58 g cm−3. When the target furnace temperature was set at 700 °C, the specimen with Ti and Al in a 1 : 3 ratio possessed the highest open porosity of 56.27% and the lowest density of 1.44 g cm−3. The porous specimens with Ti and Al in a 1 : 3 ratio exhibited the best resistance to oxidation at 650 °C in air.

 Please wait while we load your content...
Please wait while we load your content...