Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A BODIPY-based fluorescent probe for the differential recognition of Hg(II) and Au(III) ions†
Ceren
Cantürk
,
Muhammed
Üçüncü
and
Mustafa
Emrullahoğlu
*
Department of Chemistry, Faculty of Science, İzmir Institute of Technology, Urla 35430, Izmir, Turkey. E-mail: mustafaemrullahoglu@iyte.edu.tr
First published on 24th March 2015
Abstract
We describe the design, synthesis and spectral behaviour of a fluorescent molecular sensor able to recognize Hg2+ and Au3+ ions via different emission modes. The molecular sensor is constructed on a single BODIPY dye appended with a semithio-carbazone functionality as a recognition motif.
In recent years, research on the development of molecular sensors for analysing diverse biologically and environmentally important analyte species has increased.1 While the vast majority of sensors addressed in such literature are designed to recognize a specific target, less common are sensors capable of differentiating multiple targets. Differential detection of multiple analyte species can be achieved best by recognizing each species through a different signal output (i.e., emission wavelength).2 Incorporating multiple binding motifs onto a single signal-transducing molecule (chromophore/fluorophore) and, as an alternative, combining different transducing molecules have both appeared as efficient routes for sensors with multiple output modes.3
Despite recent advances in the field, it remains a challenge to differentiate metal species with similar chemical natures. For example, the ionic species of gold (Au3+) and mercury (Hg2+) share several similarities in terms of binding properties, since both have strong binding affinities toward sulphur species. When accumulated in the biological system, they thus have great potential to interact with sulphur-bearing biomolecules such as enzymes, proteins, and DNA. As a result, these metal species can disturb a series of cellular processes that cause toxicity in humans.3,4 Tracking these metal species in a living environment with the aid of a fluorescent molecular sensor is thus crucial to evaluating their roles in certain biological processes.
In their structure, most molecular sensors devised for mercury ions use sulphur moieties as a recognition motif.5 As such, it is always possible that gold and mercury species interfere with each other during their analysis, which could primarily explain why molecular sensors able to differentiate these two metal ions are extremely rare.6 Molecular tools that can differentiate multiple analytes of a similar chemical nature (e.g., gold7 and mercury5,8 ions) are therefore clearly in high demand.
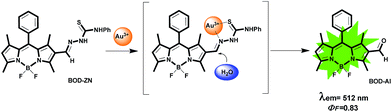
Herein, we designed a molecular sensor with a single fluorophore core appended with semithiocarbazone functionality as the metal ion recognition motif. The fluorophore core, based on a BODIPY dye,9 is designed to be inactive (i.e., non-emissive) in its initial state yet expected to become active in response to the metal species (Scheme 1). The differential detection of Hg2+ and Au3+ relies on different modulation mechanisms and can be realized with two distinct fluorescence changes, based on either an Hg2+-ligand coordination event or a gold-mediated chemical transformation.
The title compound, BOD-ZN, was prepared (40% overall) by the synthetic route outlined in Scheme 1 and, its structure was unambiguously confirmed by 1H-NMR, 13C-NMR, and HRMS spectroscopy.10
The sensing behaviour of BOD-ZN toward the addition of a range of metal ion species was studied by UV/Vis and fluorescence spectroscopy. As shown in Fig. 1a, the UV/Vis spectrum of free BOD-ZN (phosphate buffer/ethanol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, pH 7.0) displays a maximum absorption band at 533 nm, which belongs to the BODIPY chromophore. The fluorescence spectrum of BOD-ZN collected upon excitation at 460 nm exhibits a very weak emission band at 601 nm. Reasonably, BOD-ZN was nearly non-emissive, since the molecular structure of the probe bears a C
4, pH 7.0) displays a maximum absorption band at 533 nm, which belongs to the BODIPY chromophore. The fluorescence spectrum of BOD-ZN collected upon excitation at 460 nm exhibits a very weak emission band at 601 nm. Reasonably, BOD-ZN was nearly non-emissive, since the molecular structure of the probe bears a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functionality that diminishes the emission of the BODIPY core caused by a non-radiative deactivation process involving the rapid isomerization of the C
N functionality that diminishes the emission of the BODIPY core caused by a non-radiative deactivation process involving the rapid isomerization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group.
N group.
Our investigation began with the evaluation of the optical behaviour of BOD-ZN in response to the addition of Au3+ ions (e.g., AuCl3). The addition of Au3+ (1 equiv.) to BOD-ZN prompted the appearance of a new emission band at 512 nm that was assigned to the formation of a new BODIPY derivative (Fig. 1b). The appearance of this new band was accompanied with a distinct change in the solution's emission colour; the red-emitting probe solution became distinctly green, as was clearly visible to the naked eye (Fig. S19, ESI†).
The compound displaying such green emission was isolated and further characterized as BOD-AL, the hydrolysis product of BOD-ZN (Scheme 2). Evidently, the recognition of Au3+ was based on an Au3+-mediated hydrolysis reaction that resulted in the formation of a highly emissive BODIPY derivative (BOD-AL).
A systematic titration of BOD-ZN with Au3+ reveals that emission band intensity increases linearly with the increase in concentration of Au3+ in the range of 0.1–100 μM (Fig. S6, ESI†). At the same time, the kinetic study showed that the spectral response toward the addition of Au3+ was rapid (<1 min) and that the emission intensity plateaued within 20 min. Due to the addition of 10 equiv. of Au3+, which thereby enhanced intensity by over 200-fold. Moreover, the minimum amount of Au3+ detectable was evaluated to be 128.0 nM based on the signal-to-noise ratio (S/N = 3) (Fig. S2, ESI†).
Having established that the detection of Au3+ ions relies on an irreversible chemical reaction, we investigated the selectivity profile of BOD-ZN in response to other metal species. The probe proved to be highly specific for Au3+ ions, since no change was detected in the spectrum when Au+ ions were present. Furthermore, BOD-ZN showed no spectral response to other metal ions such as Cu2+, Ag+, Zn2+, Pb2+, Ni2+, Na+, Mg2+, Li+, K+, Pd2+, Co2+, Cd2+, Ca2+, Ba2+, Fe3+, and Cr3+, except for Hg2+.
We recognized the detection of Hg2+ with a different emission output. Given the addition of Hg2+, the weakly emissive probe solution immediately (<5 s) turned profoundly yellow, possibly due to the blockage of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization via strong coordination to the nitrogen electron pair which prevents the electron transfer process, and thus enhances the fluorescence emission (Scheme 3).
N isomerization via strong coordination to the nitrogen electron pair which prevents the electron transfer process, and thus enhances the fluorescence emission (Scheme 3).
Meanwhile, in the fluorescence spectrum of BOD-ZN, a new emission band appeared at 542 nm and increased linearly with the increased concentration of Hg2+ above the range of 0.1–100 μM (Fig. 2b). The detection limit of BOD-ZN for detecting Hg2+ was 160.0 nM.
In sharp contrast to the detection of Au3+, detecting Hg2+ ions proceeded reversibly. The reversibility of the binding event between BOD-ZN and Hg2+ was confirmed by the addition of Na2S to the solution pre-treated with Hg2+, which sharply decreased the emission intensity. The regeneration of fluorescence was again made possible by introducing Hg2+ ions into the solution, and the off-on switching ability of the system with Hg2+ proved the reversibility of the process (Fig. S15, ESI†).
At the same time, the binding process of Hg2+ to BOD-ZN could be clearly followed by the aid of 1H-NMR spectroscopy. During Hg2+ incubation lasting 5 min, we observed pronounced differences in the 1H-NMR spectrum of BOD-ZN. For one, the resonance of the Ha proton signal at 8.01 ppm belonging to the hydrogen atom of the aldimine shifted to a higher frequency, while the resonance of the methyl protons (Hb and Hc) in close proximity to the recognition motif shifted to a lower frequency. Furthermore, a reorganization of the phenyl ring proton signals strongly suggests the structural modification in the phenyl thiourea motif (Fig. 3).
 | ||
| Fig. 3 (a) Proposed coordination mechanism of Hg2+ to BOD-ZN; (b) 1H-NMR of BOD-ZN in methanol-d4; (c) 1H-NMR of BOD-ZN + Hg2+ (1 equiv.) in methanol-d4. | ||
The stoichiometry of the sensing event was established by following the Benesi–Hildebrand method and, accordingly, the related binding constant was determined as 4.2 × 104 M−2.11 With HRMS analysis, we also confirmed the binding of Hg2+ ions to BOD-ZN. HRMS data of the solution (Hg2+/BOD-ZN) indicated the formation of an Hg2+ ion complex, (m/z = 1204.36841 found; 1204.36354 calc.), with a binding stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.10 Given all of the above, the structure of the binding complex is most likely that shown in Scheme 3.
2.10 Given all of the above, the structure of the binding complex is most likely that shown in Scheme 3.
Having clarified the detection of both metal species, we assessed the interference of other metal ions in the detection of Au3+ and Hg2+. Although the spectral response of BOD-ZN induced by Au3+ ions showed no interference with other metal ions, the detection of Hg2+ was disturbed in the presence of Au3+ ions. More specifically, in the presence of Au3+ and Hg2+, the fluorescence spectrum initially displayed an emission band at 542 nm for the Hg2+/BOD-ZN binding complex. However, the band disappeared within 10 min, as a new band appeared at 512 nm, which indicates that Au3+ ions also mediate the hydrolysis of the Hg2+ binding complex (Fig. S18, ESI†). Notably, the same sensing behaviour was also observed by adding Au3+ ions to a solution pre-treated with Hg2+.
Relying on the impressive sensing properties of BOD-ZN, we next investigated its capacity for imaging Hg2+ and Au3+ ions in living cells. As Fig. 4a and a′ show, the images of human lung adenocarcinoma (A549) cells incubated with BOD-ZN did not display any fluorescence until the addition of the metal species. However, upon incubation with Au3+ or Hg2+, the cells started to emit a distinct fluorescence emission consistent with results obtained in the solution. Based on the nucleus staining experiment using DAPI as the staining dye, we concluded that the probe passes through the cell membrane and detects both metal species from within the cell. This preliminary cell imaging study suggested that BOD-ZN can be used efficiently for the in vitro imaging of Au3+ and Hg2+ species in living cells.
Conclusions
To close, we have studied the design, spectral behaviour, and cell-imaging capacity of a unique fluorescent molecular structure that can efficiently differentiate Hg2+ and Au3+ ions. The differential detection of Hg2+ and Au3+ was recognized in two distinct fluorescence changes: one resulting from a reversible Hg2+/sensor complex formation, the other an irreversible Au3+-mediated hydrolysis reaction. This novel molecular structure displayed the ability to recognize both metal species at nanomolar levels.Acknowledgements
We thank İzmir Institute of Technology (İZTECH) and TÜBİTAK for financial support.Notes and references
- For recent reviews, see: (a) M. E. Jun, B. Roy and K. H. Ahn, Chem. Commun., 2011, 47, 7583–7601 RSC; (b) H. Zheng, X.-Q. Zhan, Q.-N. Bian and X.-J. Zhang, Chem. Commun., 2013, 49, 429–447 RSC; (c) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed; (d) K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed; (e) X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590–659 CrossRef CAS PubMed.
- (a) L. Xue, Q. Liu and H. Jiang, Org. Lett., 2009, 11, 3454–3457 CrossRef CAS PubMed; (b) V. Luxami, Renukamal, K. Paul and S. Kumar, RSC Adv., 2013, 3, 9189–9192 RSC; (c) H. Komatsu, T. Miki, D. Citterio, T. Kubota, Y. Shindo, Y. Kitamura, K. Oka and K. Suzuki, J. Am. Chem. Soc., 2005, 127, 10798–10799 CrossRef CAS PubMed; (d) M. Yuan, W. Zhou, X. Liu, M. Zhu, J. Li, X. Yin, H. Zheng, Z. Zuo, C. Ouyang, H. Liu, Y. Li and D. Zhu, J. Org. Chem., 2008, 73, 5008–5014 CrossRef CAS PubMed; (e) D. Maity and T. Govindaraju, Chem. Commun., 2012, 48, 1039–1041 RSC; (f) P. N. Basa and A. G. Sykes, J. Org. Chem., 2012, 77, 8428–8434 CrossRef CAS PubMed; (g) L. Cao, J. Cia, Y. Huang, Q. Zhang, N. Wang, Y. Xue and D. Du, Tetrahedron Lett., 2014, 55, 4062–4066 CrossRef CAS; (h) N. R. Chereddy, P. Nagaraju, M. V. Niladri Raju, K. Saranraj, S. Thennarasu and V. J. Rao, Dyes Pigm., 2015, 112, 201–209 CrossRef CAS; (i) L. Fan, J.-C. Qin, T.-R. Li, B.-D. Wang and Z.-Y. Yang, Sens. Actuators, B, 2014, 203, 550–556 CrossRef CAS.
- I. Onyido, A. R. Norris and E. Buncel, Chem. Rev., 2004, 104, 5911–5929 CrossRef CAS PubMed.
- E. Nyarko, T. Hara, D. J. Grab, A. Habib, Y. Kim, O. Nikolskaia, T. Fukuma and M. Tabata, Chem.-Biol. Interact., 2004, 148, 19–25 CrossRef CAS PubMed.
- (a) Y.-K. Yang, K.-J. Yook and J. Tae, J. Am. Chem. Soc., 2005, 127, 16760–16761 CrossRef CAS PubMed; (b) J.-S. Wu, I.-C. Hwang, K. S. Kim and J. S. Kim, Org. Lett., 2007, 9, 907–910 CrossRef CAS PubMed; (c) G.-Q. Shang, X. Gao, M.-X. Chen, H. Zheng and J.-G. Xu, J. Fluoresc., 2008, 18, 1187–1192 CrossRef CAS PubMed; (d) S. Atilgan, T. Ozdemir and E. U. Akkaya, Org. Lett., 2010, 12, 4792–4795 CrossRef CAS PubMed; (e) Q. Zhang, X.-J. Huang and Z.-J. Zhu, RSC Adv., 2013, 3, 24891–24895 RSC; (f) S. Chen, P. Wang, C. Jia, Q. Lin and W. Yuan, Spectrochim. Acta, Part A, 2014, 133, 223–228 CrossRef CAS PubMed; (g) H. N. Kim, S.-W. Nam, K. M. K. Swamy, Y. Jin, X. Chen, Y. Kim, S.-J. Kim, S. Park and J. Yoon, Analyst, 2011, 136, 1339–1343 RSC.
- (a) M. Dong, Y.-W. Wang and Y. Peng, Org. Lett., 2010, 12, 5310–5313 CrossRef CAS PubMed; (b) E. Karakuş, M. Üçüncü and M. Emrullahoğlu, Chem. Commun., 2014, 50, 1119–1121 RSC.
- (a) M. J. Jou, X. Chen, K. M. K. Swamy, H. N. Kim, H.-J. Kim, S. G. Lee and J. Yoon, Chem. Commun., 2009, 7218–7220 CAS; (b) O. A. Egorova, H. Seo, A. Chatterjee and K. H. Ahn, Org. Lett., 2010, 12, 401–403 CrossRef CAS PubMed; (c) Y. K. Yang, S. Lee and J. Tae, Org. Lett., 2009, 11, 5610–5613 CrossRef CAS PubMed; (d) J. Y. Choi, G.-H. Kim, Z. Guo, H. Y. Lee, K. M. K. Swamy, J. Pai, S. Shin, I. Shin and J. Yoon, Biosens. Bioelectron., 2013, 49, 438–441 CrossRef PubMed; (e) J.-B. Wang, Q.-Q. Wu, Y.-Z. Min, Y.-Z. Liu and Q.-H. Song, Chem. Commun., 2012, 48, 744–746 RSC; (f) M. Üçüncü and M. Emrullahoğlu, Chem. Commun., 2014, 50, 5884–5886 RSC; (g) H. Seo, M. E. Jun, O. A. Egorova, K. H. Lee, K. T. Kim and K. H. Ahn, Org. Lett., 2012, 14, 5062–5065 CrossRef CAS PubMed; (h) M. Emrullahoğlu, E. Karakuş and M. Üçüncü, Analyst, 2013, 138, 3638–3641 RSC; (i) S. Singha, D. Kim, H. Seo, S. W. Cho and K. H. Ahn, Chem. Soc. Rev., 2015 10.1039/c4cs00328d.
- For recent reviews, see: (a) M. J. Culzoni, A. Muñoz de la Peña, A. Machuca, H. C. Goicoechea and R. Babiano, Anal. Methods, 2013, 5, 30–49 RSC; (b) Z. Yan, M.-F. Yuen, L. Hu, P. Sun and C.-S. Lee, RSC Adv., 2014, 4, 48373–48388 RSC; (c) P. Mahato, S. Saha, P. Das, H. Agarwalla and A. Das, RSC Adv., 2014, 4, 36140–36174 RSC.
- For recent reviews, see: (a) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC; (b) R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496–501 RSC; (c) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed; (d) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- See the ESI† for more details.
- (a) H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS; (b) J. Hatai, S. Pal, G. P. Jose, T. Sengupta and S. Bandyopadhyay, RSC Adv., 2012, 2, 7033–7036 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Absorbance, fluorescence and characterization data and all experimental procedures. See DOI: 10.1039/c5ra04015a |
| This journal is © The Royal Society of Chemistry 2015 |