Electrodeposited p-type Co3O4 with high photoelectrochemical performance in aqueous medium
Abstract
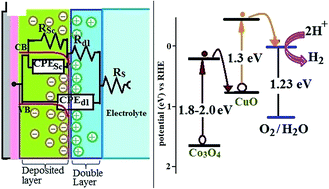
p-Type Co3O4 photocathodes with different amounts of CuO, were synthesized on fluorine doped tin oxide (FTO) via electrodeposition from a chloride bath containing suspended starch particles. All of the fabricated samples were photoresponsive toward water splitting in 0.5 M Na2SO4 under simulated sun-light. The PEC performance was evaluated using LSPV, chronoamperometry, and EIS techniques. The samples fabricated via the electrodeposition/anodizing/annealing process showed greater photocurrent response compared to the electrodeposition/annealing process. Among all the samples, the sample with an atomic composition% of Co: 24.9, Cu: 25.0 and O: 50.1 showed an optimum photocurrent response (∼6.5 mA cm−2 vs. SCE at −0.3 V). The structure, morphology/composition and optical response were characterized by XRD, FESEM/EDX and UV-Vis techniques, respectively.

 Please wait while we load your content...
Please wait while we load your content...