Effect of Keggin-type heteropolyacids on the hydrocracking of Jatropha oil
Kai Fana,
Jing Liub,
Xiaoyi Yangc and
Long Rong*a
aKey Laboratory for Biomechanics and Mechanobiology of Ministry of Education, School of Biological Science and Medical Engineering, Beihang University, Beijing 100191, P. R. China. E-mail: ronglong@buaa.edu.cn; Fax: +86 10 8233 9157; Tel: +86 10 8233 9157
bBeijing Key Laboratory of Lignocellulosic Chemistry, Beijing Forestry University, Beijing 100083, P. R. China
cEnergy and Environment international center, Beihang University, Beijing 100191, P. R. China
First published on 30th March 2015
Abstract
To investigate the effect of Keggin-type heteropolyacids on the hydrocracking of Jatropha oil, Ni-heteropolyacid/nano-hydroxyapatite (Ni-HPA/nHA) catalysts were prepared with different Keggin-type heteropolyacids loaded: phosphotungstic acid (HPW), phosphomolybdic acid (HPMo), silicotungstic acid (HSiW) and silicomolybdic acid (HSiMo). The catalysts were characterized using N2 adsorption–desorption, powder X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), temperature programmed desorption of ammonia (NH3-TPD) and thermogravimetric analysis (TGA). Their acidities at reaction temperature (360 °C) were determined using solid-state 1H nuclear magnetic resonance (1H NMR). The acidity order of the catalysts at reaction temperature was as follows: HSiW > HPW > HSiMo > HPMo. The Jatropha oil and hydrocracking product oil were analysed using liquid-state 13C nuclear magnetic resonance (13C NMR). The hydrocracking conversions of Jatropha oil over these Ni-HPA/nHA catalysts were 100%; the highest iso/n-alkane ratio was 1.64 and the lowest pour point of the product oil was −32 °C at 360 °C, 3 MPa, H2/oil (v/v) = 600 and LHSV = 2 h−1 over the Ni-HSiW/nHA catalyst. The results demonstrated that Keggin-type heteropolyacids could improve the conversion of Jatropha oil; the isomerization of hydrocracking was affected by the acidity of the heteropolyacids at reaction temperature and stronger acidity could produce more iso-alkanes.
Introduction
Biofuels, as promising renewable and sustainable alternative fuels for the replacement of limited fossil fuels, have attracted a lot of attention.1–3 Among the current production techniques of biofuel, the hydrocracking of vegetable oil plays an important role from an economic point of view.4,5 Its production has similar components to fossil fuels. Moreover, it contains few sulphur and nitrogen compounds.6,7 As a consequence, this kind of biofuel could reduce the more concerning environmental pollution and the greenhouse effect. Therefore, there are essential needs for the hydrocracking of vegetable oil.Traditional catalysts for hydrocracking were nickel-based catalysts, with sulfurated nickel and another assisting metal (W and Mo) loaded into a catalyst support (Al2O3 and SiO2).5,8,9 However, during the hydrocracking process, the sulfur-containing product oil and H2S gas emission led to contamination of the environment.10,11 On the other hand, the main content of the product oil was n-alkane, which has a relatively high pour point, the low temperature fluidity of which limited its application.12,13 Since the low temperature fluidity of a biofuel can be improved by increasing the content of iso-alkane, some research has focused on the isomerization of n-alkane. For example, NiW/SiO2–Al2O3 and Pt/SAPO-11 were used for the hydrocracking of n-decane.14,15 In previous work,16 we have found that a nano-hydroxyapatite (nHA) support with phosphotungstic acid loading could get rid of sulphur and increase the iso-alkane content to lower the pour point in the hydrocracking of Jatropha oil, demonstrating that Keggin-type heteropolyacids (HPAs) have the potential to be promising catalysts for hydrocracking. Due to their unique physicochemical properties and their wide use as homogeneous and heterogeneous acid and oxidation catalysts, it is necessary to research Keggin-type heteropolyacids in detail.
According to classical carbenium ion theory,17 the isomerization in the hydrocracking process is due to the formation of carbenium ion intermediates which require a proton donor, thus the acidity of the catalyst becomes one of the key factors for isomerization. The acidity of bifunctional catalysts is provided by the support (HY, HZSM and SAPO), and much research has demonstrated that both the acidity and structure of the catalyst support affect the isomerization.18,19 Being used in catalysis, Keggin-type heteropolyacids have a significantly higher Brønsted acidity for isomerization, compared with other types of heteropolyacids, and the Keggin-type heteropolyacids are thermally more stable.20,21 However, the results of Thomas et al. stated that the acidity of phosphotungstic acid can be changed by high temperature variation.22 Thus in this work, Keggin-type heteropolyacids, including phosphotungstic acid (HPW), phosphomolybdic acid (HPMo), silicotungstic acid (HSiW) and silicomolybdic acid (HSiMo) shall be loaded into nickel based nHA for the hydrocracking of Jatropha oil, avoiding the interference of the catalyst structure. When approaching the catalyst state of reaction, solid-state 1H NMR was applied to simulate the acidity of the catalyst at reaction temperature (360 °C). The different hydrocracking results of the Ni-HPA/nHA catalysts were also compared and discussed.
Experimental
Catalyst preparation and characterization
The nHA was prepared by a precipitation method.23 All of the chemical reagents used in this work were analytical reagents and were purchased from Sinopharm Chemical Reagent Co., Ltd, China. Ca(NO3)2·4H2O was dissolved in deionized water. Then Na3PO4·6H2O was dissolved in deionized water to obtain Na3PO4 solution. The Na3PO4 solution was titrated into the blend solution at a Ca/P molar ratio of 1.67 with mechanical stirring and ultrasonic processing at pH 10 at room temperature. After that, the stirring was continued for 1 h with ultrasonic processing, and then the slurry was aged for 12 h, and washed with deionized water to pH 7. The obtained precipitate after centrifugation was calcined at 400 °C for 6 h.Then, the Ni-HPA/nHA catalysts were prepared by impregnation of aqueous solutions of Ni(NO3)2·6H2O, and impregnated aqueous solutions of the heteropolyacids. Impregnated samples were dried at 105 °C overnight and calcined at 200 °C for 6 h. The obtained catalyst samples were Ni-HPW/nHA, Ni-HPMo/nHA, Ni-HSiW/nHA and Ni-HSiMo/nHA. We set the HPW loading amount to 30% (which was confirmed as the suitable HPW loading amount for hydrocracking from our previous work) as a reference.16 To guarantee that the different catalyst samples had the same number of moles of heteropolyacid for comparison, the different heteropolyacid loading amounts were as follows: H3PW12O40 (30 wt%), H3PMo12O40 (19 wt%), H4SiW12O40 (30 wt%) and H4SiMo12O40 (19 wt%), the Ni loading amount was 5 wt%.
N2 adsorption–desorption was measured to determine specific surface areas and pore size distributions, using a V-Sorb 2800 TP surface area and pore distribution analyzer instrument (Beijing Gold APP Instruments Co., Ltd). The Ni-HPA/nHA catalyst samples were degassed under vacuum at 300 °C for 2 h before the measurements. Specific surface areas were determined using the Brunauer–Emmett–Teller (BET) procedure. The area of the micropores and pore size distributions (pore diameters and pore volumes) were respectively determined using the t-plot method and Barrett–Joyner–Halenda (BJH) method from the adsorption branch of the isotherms.
X-ray diffraction (XRD) patterns were measured using Cu-Kα radiation at 40 kV and 30 mA, recording on a D/max2500VB2+/PC XRD analyzer (Japan Electronics Science Co., Ltd.). The Ni-HPA/nHA catalyst samples were measured in the 2θ range from 10° to 80° at a scan speed of 2° min−1.
The metal state of the Ni-HPA/nHA catalyst samples was determined from X-ray photoelectron spectroscopy (XPS) measurements, using a ESCALAB 250Xi instrument (Thermofisher) at 280 eV pass energy. Binding energies were corrected for sample charging using the C 1s peak at 284.6 eV for adventitious carbon as a reference.
The acidities of the Ni-HPA/nHA catalyst samples were determined from the temperature programmed desorption of ammonia (NH3-TPD) using a DAS-7000 Multi-functional automatic Adsorption Instrument (Beijing surface technology Co., Ltd). All samples were pretreated in He (25 mL min−1) at 300 °C for 2 h. After adsorption of ammonia, the desorption step was performed at a heating rate of 10 °C min−1 from 100 °C to 700 °C.
These Ni-HPA/nHA catalysts samples were also studied using solid-state 1H NMR (Switzerland Bruker AVANCE III nuclear magnetic resonance spectrometer) as a function of the temperature of 20 °C and 360 °C (which was confirmed as the suitable reaction for hydrocracking by our previous work).16 Each Ni-HPA/nHA catalyst powder (200 mg) was treated under vacuum at a temperature of 20 °C and 360 °C for 2 h in a sealed ampoule. In order to achieve relatively quantitative results, all of the 1H NMR spectra were recorded at the same time. Each ampoule, containing a Ni-HPA/nHA catalyst sample, was broken in a glovebox, then the rotor was filled and tightly closed by using a cap. The amount of catalyst sample was determined by weighing the rotor before and after it was filled. The NMR spectra were recorded on a spectrometer operating at 300.13 MHz, the spinning rate was 10 kHz and the eight scans were acquired after a π/2 pulse.22
The amount of coke on the past-reacted catalysts was determined using thermogravimetric analysis (TGA), performed on a NETZSCH STA449F3 analyzer. Samples were first heated from 30 °C to 550 °C with a heating rate of 30 °C min−1 in a N2 flow of 100 mL min−1, maintained at a temperature of 550 °C for 15 min, then heated linearly at 30 °C min−1 to 800 °C in 100 mL min−1 O2 flow. The weight loss of the samples was calculated by microcomputer.
Catalytic activity measurements
The Jatropha oil was purchased from Jiangsu Donghu Bioenergy Co., Ltd, including myristic acid (0.8%), arachic acid (0.5%), linolenic acid (0.9%), palmitoleic acid (1.2%), stearic acid (7.3%), palmitic acid (14.8%), linoleic acid (36.2%) and oleic acid (38.3%).The experiments were performed in a fixed-bed reactor (JF-2, Tianjin Golden Eagle Technology Co., Ltd, China), including feed system, heating section, tubular reactor, condensation section, storage section, instrumentation and control section. The reaction temperature, system pressure and hydrogen input rate were controlled by the microcomputer.
The different catalyst samples (10 g) were loaded into the tubular reactor and activated prior to the experiments with a H2 flow at 400 °C and 3 MPa for 3 h. The reaction conditions for the catalytic hydrocracking experiments were a temperature of 360 °C, a pressure of 3 MPa, LHSV 2 h−1, and a H2 to feed ratio of 600 mL H2 gas per mL liquid feed.16
After 8 h of stabilization of the reaction conditions, the product oil was analyzed using a gas chromatograph equipped with a flame-ionization detector (FID). The capillary column (AT.SE-30, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences) dimensions were 0.32 mm i.d. × 30 m with a film thickness of 0.5 μm. Normal alkane standards (purchased from Sigma-Aldrich, LLC) were used to estimate the relative percentages and distributions of the product oil with respect to their carbon numbers. The conversion of Jatropha oil was calculated as:
| C = 100% − C(T) | (1) |
The content of n-alkane and iso-alkane was calculated on the basis of GC analyzer program data on a microcomputer. To evaluate the low temperature fluidity, the pour point of the product oil was determinated (based on ASTM D97-93) by a pour point tester (Normalab, model NTE 450, France). To analyse the chemical components of the Jatropha oil and liquid hydrocracking products, the 13C NMR spectra for the liquid products were recorded using a Varian (USA) INOVA 500 NMR spectrometer operating at a frequency of 125 MHz, using a 5 mm inverse Z-gradient vitreous probe.
Results and discussion
Characterization of the catalysts
The pore size distribution and nitrogen adsorption–desorption isotherms of the different catalyst samples are presented in Fig. 1. The pore size distribution of nHA mainly ranged from 5 to 50 nm, identifying it as mesoporous. Based on the literature, the mesoporous material support was supposed to be appropriate for the catalysis of a macromolecule, such as Jatropha oil. The N2 adsorption volume of all the catalyst samples increased gradually as the relative pressure increased and then increased rapidly at high relative pressure (P/P0 > 0.45) in agreement with the literature.24 After impregnation, the total adsorption of the samples had no obvious change. As shown in Table 1, the nHA also exhibited a large specific surface area (109 m2 g−1) and total pore volume (0.51 cm3 g−1). After impregnation, the specific surface areas of and total pore volumes of the catalyst samples increased while the average pore diameters slightly decreased, suggesting that some of the pores were blocked by metal and heteropolyacids.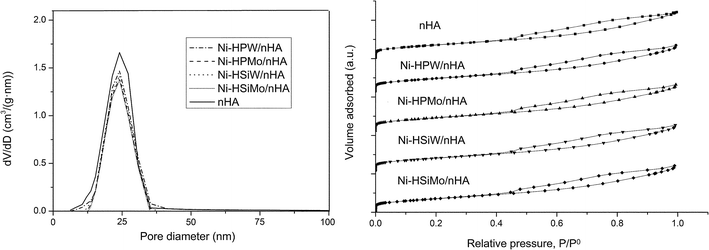 | ||
| Fig. 1 Pore size distributions and nitrogen adsorption–desorption isotherms of the catalyst samples. | ||
| Catalyst | Specific surface area, m2 g−1 | Total pore volume, cm3 g−1 | Average pore diameter, nm |
|---|---|---|---|
| nHA | 109 | 0.51 | 22 |
| Ni-HPW/nHA | 97 | 0.47 | 20 |
| Ni-HPMo/nHA | 98 | 0.46 | 20 |
| Ni-HSiW/nHA | 96 | 0.48 | 20 |
| Ni-HSiMo/nHA | 99 | 0.46 | 20 |
The XRD patterns of the different catalyst samples are shown in Fig. 2. All of the catalyst samples exhibited the same characteristic peaks for nHA at 2θ = 25.8°, 31.9°, 39.8°, 46.7° and 49.5°.25,26 After Ni loading, the characteristic peaks for a Ni oxide phase were observed at 2θ = 37.3°, 43.6°, and 63.4°.23,27 Then, after the heteropolyacid loadings, in each of the ranges of 2θ = 16–23°, 25–30° and 31–38°, the catalyst samples showed characteristic peaks for heteropolyacids with Keggin-type structures.28
 | ||
| Fig. 2 XRD patterns of (a) nHA, (b) Ni/nHA, (c) Ni-HPW/nHA, (d) Ni-HPMo/nHA, (e) Ni-HSiW/nHA and (f) Ni-HSiMo/nHA. | ||
In our previous work,16 we had proved that phosphotungstic acid could reduce Ni2+ to Ni0, which contributed to the enhancement of the hydrogenation activity. The same result was also reported by Zheng et al.29 To investigate whether the oxidation state of Ni was affected by other heteropolyacids, the different catalyst samples were examined using X-ray photoelectron spectroscopy (XPS). The Ni 2p core level signal of the catalyst is shown in Fig. 3. The binding energy values at 851.4 and 852.1 eV were associated with the Ni0 of the Ni/nHA and Ni-HPA/nHA catalysts respectively, and the other peaks were associated with Ni2+.30 It was obvious that all of the heteropolyacids had a reducing capacity, being able to increase the amount of Ni0. Based on the reported literature,31,32 the reduced metal was able to promote the activity of the hydrogenation, thus the conversion of Jatropha oil was improved.
The NH3-TPD profiles of the Ni-HPA/nHA catalysts with different Keggin-type heteropolyacids loaded are shown in Fig. 4. The total acidity of the catalysts are recorded in Table 2. The nHA support only represented a small peak at around 200 °C. After heteropolyacid loading, all of the Ni-HPA/nHA catalysts exhibited two broad peaks at around 200 °C and 450 °C. These results illustrated that Keggin-type heteropolyacid loading increases the acidity (including weak and strong acidity) of the catalysts, offering acid sites for catalysis, then as the proton source, the heteropolyacid could promote the isomerization process of hydrocracking.14,33 However, different kinds of Ni-HPA/nHA catalysts also represented a difference in the acidity. It can be seen from Table 2 that the order of acidity is: HPW > HSiW > HPMo > HSiMo, in accordance with reported literature.20,28
| Catalyst | Total acidity (mmol g−1) |
|---|---|
| nHA | 0.11 |
| Ni-HPW/nHA | 0.81 |
| Ni-HSiW/nHA | 0.65 |
| Ni-HPMo/nHA | 0.52 |
| Ni-HSiMo/nHA | 0.43 |
To investigate the acidity of the catalysts at the hydrocracking reaction temperature (360 °C), solid-state 1H NMR was used. Fig. 5 shows the 1H NMR spectra of these Ni-HPA/nHA catalyst samples as a function of temperature at 20 °C and 360 °C. In each case, the spectra exhibited two main peaks, a sharp one at around 0 ppm represented the structural hydroxyl group of nHA,34 and another broad resonance between 2.5 and 15 ppm corresponded to the protons of the heteropolyacids.35 After integration of the relative peak area of the Ni-HPA/nHA catalysts, we set the area for HPW and the hydroxyl group in Ni-HPW/nHA at 20 °C to 100%, then the profile was recorded in Table 3. It was obvious that at 20 °C, the order for the proton content of these Ni-HPA/nHA catalyst samples was in agreement with the NH3-TPD profiles. At 360 °C, the signal for the nHA hydroxyl group remained almost the same, illustrating that the reaction temperature had no effect on the acidity of the nHA support. However, there was a reduction in the proton content for all of the heteropolyacids. HPW and HPMo lost more protons than HSiW and HSiMo, and the new order of the acidity at 360 °C was as follows: HSiW > HPW > HSiMo > HPMo. These results demonstrated that the acidity of Keggin-type heteropolyacids was affected by the high temperature, that the structures of the heteropolyacids were probably changed or damaged, and that the heteropolyacids whose heteroatoms were silicon had better high temperature resistance than the heteropolyacids whose heteroatoms were phosphorus. The influence of the Ni-HPA/nHA catalysts acidity variation on the hydrocracking results shall be discussed later.
 | ||
| Fig. 5 Solid-state 1H NMR spectra of Ni-HPA/nHA catalysts with different Keggin-type heteropolyacids loaded at 20 °C and 360 °C. | ||
| Catalyst | Relative peak area of HPAs (%) | Relative peak area of hydroxyl group (%) | ||
|---|---|---|---|---|
| 20 °C | 360 °C | 20 °C | 360 °C | |
| Ni-HPW/nHA | 100 | 51.8 | 100 | 100 |
| Ni-HSiW/nHA | 81.6 | 63.3 | 100 | 100 |
| Ni-HPMo/nHA | 64.1 | 24.8 | 100 | 100 |
| Ni-HSiMo/nHA | 48.7 | 32.5 | 100 | 100 |
According to the reference ISO 6964-1986, the amount of carbonaceous deposition (coke) on the used catalysts was determined by TGA. As shown in Fig. 6, the amount of coke on these Ni-HPA/nHA catalyst samples after use for 8 h was significantly different, following the order: HSiW > HPW > HSiMo > HPMo. On the other hand, after use for 148 h, the amount of coke on these Ni-HPA/nHA catalyst samples was also different, increasing by 0.71%, 0.65%, 0.44% and 0.43% compared to the amounts at 8 h, respectively. Based on the literature, the amount of coke on the hydrocracking catalyst was related to the acidity of the catalyst, which could donate a proton for the coke formation.36–38 Therefore, the stronger the acidity of the catalyst, the more coke was obtained.
 | ||
| Fig. 6 TGA profiles of a Ni-HSiW/nHA, b Ni-HPW/nHA, c Ni-HSiMo/nHA, and d Ni-HPMo/nHA after use for (a) 8 h and (b) 148 h at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1. | ||
As the 1H NMR spectra confirmed, the acidities of these Ni-HPA/nHA catalyst samples at 360 °C reduced, which would explain why the coke amount on these catalysts was not as much as in the reported literature, where the reaction temperature was lower than 360 °C.39,40 Especially for HPW and HPMo, their acidities were significantly influenced by the high temperature, limiting their capacity for proton donation. Therefore, the amount of coke in these Ni-HPA/nHA catalyst samples after use for 8 h and 148 h was in the same order as the catalyst acidities at 360 °C.
Hydrocracking of Jatropha oil
The GC charts of the product oil from the hydrocracking of Jatropha oil over the different catalyst samples at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1 are shown in Fig. 7. The main products were alkanes (including iso-alkane and n-alkane) ranging from C15–C18.16 The distribution of the product oil is shown in Fig. 8. It was obvious that the distribution of the product oil was significantly influenced by the different Ni-HPA/nHA catalysts. After heteropolyacid loading, the GC charts and distribution of the product oil all confirmed that the content of the diesel range alkanes (C10–C20) and gasoline range alkanes (<C10)41 had a close relationship with the acidity of the Ni-HPA/nHA catalysts at 360 °C. The Ni-HSiW/nHA catalyst lead to more gasoline range alkanes and less diesel range alkanes, while Ni-HPMo/nHA lead to more diesel range alkanes and less gasoline range alkanes.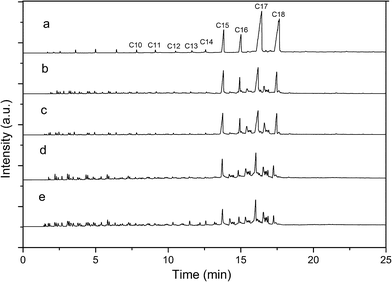 | ||
| Fig. 7 GC charts of the liquid products over (a) Ni/nHA, (b) Ni-HPMo/nHA, (c) Ni-HSiMo/nHA, (d) Ni-HPW/nHA and (e) Ni-HSiW/nHA at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1. | ||
 | ||
| Fig. 8 Distribution of the product oil over the different catalyst samples at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1. | ||
The process of the hydrocracking of Jatropha oil is shown in Fig. 9.16,41 Chemically, 3 moles of fatty acid are produced from 1 mole of raw materials (triglycerides). As the literature has proves that after the oxygen removal reaction in a hydrogen atmosphere, the fatty acid transformed to an alkane. There are two routes for oxygen removal, one is hydrodeoxygenation (HDO), whose products are even carbon number alkanes (mainly C16 and C18) and H2O; the other is hydrodecarboxylation (HDC), including decarbonylation and decarboxylation, whose products are odd carbon number alkanes (mainly C15 and C17) and CO and CO2. Regardless of the reaction conditions, this reaction is mainly due to the hydrogenation capacity, which mainly depends on the metallicity of the catalyst.
After that, the isomerization process of the alkanes happens by the cooperation of metal and acid sites.42 For the catalysts in this research, these were offered by Ni and the heteropolyacids. According to the classical carbenium ion principle, the isomerization process was as follows: (I) through hydride elimination, the n-alkane transforms to n-olefin. (II) The carbenium ion intermediates were formed by proton addition. (III) The formed normal carbenium ion intermediates isomerized to branched carbenium ion intermediates. (IV) Iso-olefin was formed by eliminating a proton of the carbenium ion intermediates. (V) Through hydrogenation, the iso-olefin was transformed to iso-alkane. The metal sites achieved steps 1 and 5, while the acid sites achieved the other steps. Therefore, as the GC charts illustrate (see Fig. 7), the main content of the product oil was n-alkanes and iso-alkanes ranging from C15–C18. However, at step 3, the carbenium ion intermediates still had the possibility to crack. If the cracking reaction was enhanced, more gasoline range alkanes could be obtained.
The properties of the product oil obtained from the hydrocracking of Jatropha oil over Ni-HPA/nHA catalysts with different heteropolyacids loaded at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1 are shown in Table 4. All of the Ni-HPA/nHA catalysts had the similar function of improving the catalytic activity of the hydrocracking, and their conversions achieved 100%. Based on the characterization data from XPS and NH3-TPD, the heteropolyacids increased the Ni0 content and offered protons for isomerization, therefore the hydrocracking activity was improved and more iso-alkanes were obtained.14,31–33,43 Through the comparison of different Ni-HPA/nHA catalysts, it was obvious that the Ni-HSiW/nHA catalyst produced more iso-alkanes, achieving the highest iso/n ratio (1.78) and lowest pour point (−32 °C). The iso/n ratio order was the same as the acidity of these catalysts at 360 °C. Based on the 1H NMR spectra and the results reported by Zhang et al.,44 the increased acidity of the catalyst leads to more formation of carbenium ion intermediates, enhancing both the isomerization and cracking, eventually producing more iso-alkanes and gasoline range alkanes. The profiles for the product oil distribution and iso/n ratio were in agreement with it. Besides, the ratio of (C15 + C17)/(C16 + C18) also increased and had an almost linear correlation with the acidity of the catalyst, suggesting the strong acidity tendency of the HDC route.
| Catalyst | Conversion (%) | (C15 + C17)/(C16 + C18) | Iso/n ratio | Pour point (°C) |
|---|---|---|---|---|
| Ni/nHA | 84.6 | 1.1 | 0.3 | 10 |
| Ni-HPMo/nHA | 100 | 2.2 | 1.41 | −18 |
| Ni-HSiMo/nHA | 100 | 2.8 | 1.52 | −23 |
| Ni-HPW/nHA | 100 | 4.4 | 1.64 | −28 |
| Ni-HSiW/nHA | 100 | 4.8 | 1.78 | −32 |
The analysis of the 1H NMR spectra showed the variation in the acidity of the Ni-HPA/nHA catalysts at the reaction temperature (360 °C). Consequently the results (iso/n ratio and distribution of the product oil) for these Ni-HPA/nHA catalysts were different from other experimental results with relatively low reaction temperatures.33,40 This result was also meaningful for the catalysis of heteropolyacid catalysts used at high temperature conditions. Moreover, a different distribution of product oil can be obtained by loading different heteropolyacids, fulfilling different needs.
Analyses of liquid products
To analyse the chemical changes of the Jatropha oil and the product oil of the hydrocracking, liquid-state 13C NMR was used. The 13C NMR profiles of Jatropha oil and the product oils obtained over Ni-HPA/nHA catalysts with different heteropolyacids loaded at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1 are shown in Fig. 10. For Jatropha oil, the existing fatty acid chain peaks were mainly located in four areas: 10–50 ppm, 60–80 ppm, 120–140 ppm and 170–180 ppm, corresponding to C–C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.45,46 For the product oil of the hydrocracking, all of the peaks were only in the alkane region between 10–50 ppm, demonstrating the complete conversion of Jatropha oil. Based on the results of Sarpal et al., the n-alkane content was in direct proportion to the integral intensities of the signals in the 29.5–30 ppm region (representing –(CH2)n–), while the content of iso-alkane was in proportion to the integral intensities of the signals in the 10–15 ppm region (representing –CH3).47,48 Then the ratio of the –CH3 signal intensities to –(CH2)n– could be used for evaluating the ratio of iso/n-alkane. After integration and calculation, the ratio profiles of –CH3/–(CH2)n– were recorded and are shown in Table 5. After comparison of the different Ni-HPA/nHA catalysts, the highest ratio of –CH3/–(CH2)n– was obtained by hydrocracking over the Ni-HSiW/nHA catalyst. The –CH3/–(CH2)n– ratio order was in accord with the iso/n ratio of hydrocracking, demonstrating that heteropolyacid loading could enhance the isomerization capacity of the hydrocracking catalyst.
O.45,46 For the product oil of the hydrocracking, all of the peaks were only in the alkane region between 10–50 ppm, demonstrating the complete conversion of Jatropha oil. Based on the results of Sarpal et al., the n-alkane content was in direct proportion to the integral intensities of the signals in the 29.5–30 ppm region (representing –(CH2)n–), while the content of iso-alkane was in proportion to the integral intensities of the signals in the 10–15 ppm region (representing –CH3).47,48 Then the ratio of the –CH3 signal intensities to –(CH2)n– could be used for evaluating the ratio of iso/n-alkane. After integration and calculation, the ratio profiles of –CH3/–(CH2)n– were recorded and are shown in Table 5. After comparison of the different Ni-HPA/nHA catalysts, the highest ratio of –CH3/–(CH2)n– was obtained by hydrocracking over the Ni-HSiW/nHA catalyst. The –CH3/–(CH2)n– ratio order was in accord with the iso/n ratio of hydrocracking, demonstrating that heteropolyacid loading could enhance the isomerization capacity of the hydrocracking catalyst.
 | ||
| Fig. 10 The 13C NMR profiles of Jatropha oil and the product oils obtained over Ni-HPA/nHA catalysts with different heteropolyacids loaded at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1. | ||
| Catalyst | Ratio of –CH3/(–CH2)n– |
|---|---|
| Jatropha oil | 0.18 |
| Ni-HPMo/nHA | 0.91 |
| Ni-HSiMo/nHA | 1.09 |
| Ni-HPW/nHA | 1.18 |
| Ni-HSiW/nHA | 1.27 |
Reaction of time-on-stream
The conversion of Jatropha oil and the iso/n-alkane ratio of the product oils over these Ni-HPA/nHA catalyst samples as a function of the reaction time at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1 are shown in Fig. 11. It was obvious that after 8 h, both the conversion and iso/n-alkane ratio remained almost constant, suggesting that the catalytic system had reached steady state. After a 148 h reaction of time-on-stream, the conversion and ratio of iso/n-alkane still remained the same. As the characterization of the catalysts and literature all proved,14,31–33 the heteropolyacids loaded had the capacity for reduction and being the proton source, therefore, that would keep the hydrocracking process stable.Conclusion
The effect of four Keggin-type heteropolyacids loaded on an nHA support were investigated for the hydrocracking of Jatropha oil. The heteropolyacid loading could increase the reduction of Ni and provide protons for the isomerization of hydrocracking. As a result, the conversion of Jatropha oil was improved and the isomerization was enhanced. The acidity of the catalysts at reaction temperature (360 °C) was detected using solid-state 1H NMR, its order was as follows: HSiW > HPW > HSiMo > HPMo. The stronger acidity of the heteropolyacid catalysts could provide more protons for the formation of carbenium ion intermediates, promoting the isomerization of hydrocracking. Thus, among these catalysts, Ni-HSiW/nHA achieved the product oil with the highest ratio of iso/n-alkane (1.78) and the lowest pour point (−32 °C).References
- U. Rashid, F. Anwar, B. R. Moser and G. Knothe, Bioresour. Technol., 2008, 99, 8175–8179 CrossRef CAS PubMed.
- L. C. Meher, D. V. Sagar and S. N. Naik, Renewable Sustainable Energy Rev., 2006, 10, 248–268 CrossRef CAS PubMed.
- J. Hill, E. Nelson, D. Tilman, S. Polasky and D. Tiffany, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11206–11210 CrossRef CAS PubMed.
- P. Simacek, D. Kubicka, G. Sebor and M. Pospisil, Fuel, 2009, 88, 456–460 CrossRef CAS PubMed.
- R. Kumar, B. S. Rana, R. Tiwari, D. Verma, R. Kumar, R. K. Joshi, M. O. Garg and A. K. Sinha, Green Chem., 2010, 12, 2232–2239 RSC.
- A. Sivasamy, K. Y. Cheah, P. Fornasiero, F. Kemausuor, S. Zinoviev and S. Miertus, ChemSusChem, 2009, 2, 278–300 CrossRef CAS PubMed.
- B. Donnis, R. G. Egeberg, P. Blom and K. G. Knudsen, Top. Catal., 2009, 52, 229–240 CrossRef CAS.
- D. Kubička, P. Šimáček and N. Žilková, Top. Catal., 2009, 52, 161–168 CrossRef.
- R. Tiwari, B. S. Rana, R. Kumar, D. Verma, R. Kumar, R. K. Joshi, M. O. Garg and A. K. Sinha, Catal. Commun., 2011, 12, 559–562 CrossRef CAS PubMed.
- P. Priecel, D. Kubička, L. Čapek, Z. Bastl and P. Ryšánek, Appl. Catal., A, 2011, 397, 127–137 CrossRef CAS PubMed.
- M. Toba, Y. Abe, H. Kuramochi, M. Osako, T. Mochizuki and Y. Yoshimura, Catal. Today, 2011, 164, 533–537 CrossRef CAS PubMed.
- V. M. Akhmedov and S. H. Al-Khowaiter, Catal. Rev.: Sci. Eng., 2007, 49, 33–139 CAS.
- J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164–7183 CrossRef CAS PubMed.
- M. Roussel, J. L. Lemberton, M. Guisnet, T. Cseri and E. Benazzi, J. Catal., 2003, 218, 427–437 CrossRef CAS.
- R. Bertolo, J. M. Silva, F. Ribeiro, F. J. Maldonado-Hodar, A. Fernandes and A. Martins, Appl. Catal., A, 2014, 485, 230–237 CrossRef CAS PubMed.
- K. Fan, J. Liu, X. Yang and L. Rong, Int. J. Hydrogen Energy, 2014, 39, 3690–3697 CrossRef CAS PubMed.
- Y. Y. Liu, R. Sotelo-Boyas, K. Murata, T. Minowa and K. Sakanishi, Energy Fuels, 2011, 25, 4675–4685 CrossRef CAS.
- P. Mériaudeau, V. A. Tuan, V. T. Nghiem, S. Y. Lai, L. N. Hung and C. Naccache, J. Catal., 1997, 169, 55–66 CrossRef.
- K. C. Park and S. K. Ihm, Appl. Catal., A, 2000, 203, 201–209 CrossRef CAS.
- M. N. Timofeeva, Appl. Catal., A, 2003, 256, 19–35 CrossRef CAS.
- F. J. Mendez, A. Llanos, M. Echeverria, R. Jauregui, Y. Villasana, Y. Diaz, G. Liendo-Polanco, M. A. Ramos-Garcia, T. Zoltan and J. L. Brito, Fuel, 2013, 110, 249–258 CrossRef CAS PubMed.
- A. Thomas, C. Dablemont, J. M. Basset and F. Lefebvre, C. R. Chim., 2005, 8, 1969–1974 CrossRef CAS PubMed.
- G. Zhou, Y. Hou, L. Liu, H. Liu, C. Liu, J. Liu, H. Qiao, W. Liu, Y. Fan and S. Shen, Nanoscale, 2012, 4, 7698–7703 RSC.
- L. B. Kong, J. Ma and F. Boey, J. Mater. Sci., 2002, 37, 1131–1134 CrossRef CAS.
- Y. Sun, G. Guo, D. Tao and Z. Wang, J. Phys. Chem. Solids, 2007, 68, 373–377 CrossRef CAS PubMed.
- H. C. Wu, T. W. Wang, J. S. Sun, W. H. Wang and F. H. Lin, Nanotechnology, 2007, 18, 165601–165609 CrossRef.
- R. Chakraborty and S. K. Das, Ind. Eng. Chem. Res., 2012, 51, 8404–8414 CrossRef CAS.
- O. Benlounes, S. Mansouri, C. Rabia and S. Hocine, J. Nat. Gas Chem., 2008, 17, 309–312 CrossRef CAS.
- J. Zheng, Z. Wu, Z. Yan, J. Li, W. Lai, X. Yi, B. Chen, W. Fang and H. Wan, Fuel, 2013, 104, 547–552 CrossRef CAS PubMed.
- T. Lehmann, T. Wolff, V. Zahn, P. Veit, C. Hamel and A. Seidel-Morgenstern, Catal. Commun., 2011, 12, 368–374 CrossRef CAS PubMed.
- M. J. Ledoux and B. Djellouli, Appl. Catal., 1990, 67, 81–91 CrossRef CAS.
- Y. Yoshimura, H. Shimada, T. Sato, M. Kubota and A. Nishijima, Appl. Catal., 1987, 29, 125–140 CrossRef CAS.
- D. D. S. Liu, J. Monnier, G. Tourigny, J. Kriz, E. Hogan and A. Wong, Pet. Sci. Technol., 1998, 16, 597–609 CrossRef CAS.
- Q. Liu, J. R. de Wijn, K. de Groot and C. A. van Blitterswijk, Biomaterials, 1998, 19, 1067–1072 CrossRef CAS.
- A. Thomas, C. Dablemont, J.-M. Basset and F. Lefebvre, C. R. Chim., 2005, 8, 1969–1974 CrossRef CAS PubMed.
- L. Liwu, Z. Tao, Z. Jingling and X. Zhusheng, Appl. Catal., 1990, 67, 11–23 CrossRef.
- V. H. de Beer, F. J. Derbyshire, C. K. Groot, R. Prins, A. W. Scaroni and J. M. Solar, Fuel, 1984, 63, 1095–1100 CrossRef CAS.
- S. Liu, G. Xiong, S. Sheng, Q. Miao and W. Yang, Stud. Surf. Sci. Catal., 1998, 119, 747–752 CrossRef CAS.
- P. A. Nikulshin, A. V. Mozhaev, A. A. Pimerzin, V. V. Konovalov and A. A. Pimerzin, Fuel, 2012, 100, 24–33 CrossRef CAS PubMed.
- B. Katryniok, S. Paul, M. Capron, V. Belliere-Baca, P. Rey and F. Dumeignil, ChemSusChem, 2012, 5, 1298–1306 CrossRef CAS PubMed.
- Y. Liu, R. Sotelo-Boyas, K. Murata, T. Minowa and K. Sakanishi, Energy Fuels, 2011, 25, 4675–4685 CrossRef CAS.
- T. Degnan and C. Kennedy, AIChE J., 1993, 39, 607–614 CrossRef CAS PubMed.
- J. C. Yori, J. M. Grau, V. M. Benitez and J. Sepulveda, Appl. Catal., A, 2005, 286, 71–78 CrossRef CAS PubMed.
- S. Zhang, S.-L. Chen and P. Dong, Catal. Lett., 2010, 136, 126–133 CrossRef CAS PubMed.
- A. Adhvaryu, S. Z. Erhan, Z. S. Liu and J. M. Perez, Thermochim. Acta, 2000, 364, 87–97 CrossRef CAS.
- L. Mannina, G. Dugo, F. Salvo, L. Cicero, G. Ansanelli, C. Calcagni and A. Segre, J. Agric. Food Chem., 2003, 51, 120–127 CrossRef CAS PubMed.
- A. S. Sarpal, G. S. Kapur, A. Chopra, S. K. Jain, S. P. Srivastava and A. K. Bhatnagar, Fuel, 1996, 75, 483–490 CrossRef CAS.
- A. S. Sarpal, G. S. Kapur, S. Mukherjee and S. K. Jain, Fuel, 1997, 76, 931–937 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |




