Preparation of poly(methyl methacrylate)/polystyrene/poly(acrylonitrile-co-butadiene) tri-layer core–shell nanoparticles and their postpolymerization modification via catalytic latex hydrogenation†
Hui Wang,
Lijuan Yang and
Garry L. Rempel*
Department of Chemical Engineering, University of Waterloo, 200 University Ave. West, Waterloo, Ontario N2L 3G1, Canada. E-mail: grempel@uwaterloo.ca; Fax: +1 519 7464979; Tel: +1 519 8884567 ext. 32702
First published on 24th April 2015
Abstract
Poly(methyl methacrylate)/polystyrene/poly(acrylonitrile-co-butadiene) (PMMA/PS/NBR) tri-layer core–shell elastomeric nanoparticles were synthesized using a facile and robust one-pot semibatch emulsion polymerization technique which allows for scale-up for industrial production. The prepared core–shell polymer nanoparticles consist of a PMMA core, PS secondary layer, and diene-containing NBR outer shell. These nanoparticles were subsequently chemically modified via a “green” postpolymerization process, i.e., catalytic latex hydrogenation without the assistance of any organic solvent. As the hydrogenated product, PMMA/PS/HNBR (hydrogenated NBR) tri-layer core–shell nanoparticles were obtained. The rate of hydrogenation was considerably fast and a high degree of hydrogenation was achieved. Meanwhile no crosslinking was observed for the polymers after the hydrogenation process. This study is expected to extend the research relating to the synthesis of polymer nanoparticles with complex architecture and “green” chemical modification of unsaturated polymers in the aqueous phase.
Introduction
Recent years have seen considerable progress in the preparation methods, modification, functions, and applications of core–shell structured polymer nanoparticles.1–3 The interest in such nanomaterials is a result of that the core–shell nanostructures can render polymers with synergically outstanding physiochemical properties and tunable surface functionalities with molecular and supramolecular structures over their single-component counterparts when the core–shell heterogeneous polymer domains are optimally designed.4–7Core–shell polymer nanoparticles can be divided into two categories depending on the relative hardness of the core and shell polymer domains. On the one hand, a physical composition composed of a soft rubber core and a hard shell can provide corresponding polymers with enhanced strength and tough properties.8,9 The core or shell can be crosslinked depending on the requirements for specific applications. The rubber core is mostly made of a low glass transition temperature (Tg) polymer while the hard shell is usually prepared from a relatively high Tg polymer. The glassy shell can be designed to increase the chemical reactivity with the polymer matrix. These types of core–shell nanoparticles are usually synthesized to act as impact modifiers due to the fact that the rubbery core provides resistance to impact especially at low temperature, whereas the hard shell not only provides rigidity and compatibility to the polymer matrix but also keeps the particles in the desired shape and dispersibility.10,11 On the other hand, core–shell polymer nanoparticles can be fabricated using a stiff core and a soft shell, in which the stiff core is responsible for the stability of the particles while the flexible shell is required for film formation after casting on surfaces.12 These types of structured nanoparticles are commonly used as additives, coatings, paints, and binders with a high block resistance and low minimum film-forming temperature (MFFT).13,14 Their excellent film-forming properties however are difficult to achieve by physical blending of two or more different polymer components.15,16 With respect to our work, the core–shell nanoparticles we proposed to synthesize are of the second type of particles, which are constituted by a poly(methyl methacrylate)/polystyrene (PMMA/PS) stiff core–shell and a soft shell of poly(acrylonitrile-co-butadiene) (NBR) rubber. A PMMA/PS combination is designed to endow the improvements in the physicochemical properties by taking advantage of the unique properties of PMMA and PS, which for example can be used as a reinforcing filler by adjusting the ratio of PMMA and PS. The outer shell NBR or HNBR (the subsequent hydrogenated product of NBR) provides important rubber properties, especially HNBR as it is used as a high performance speciality rubber. NBR and HNBR based elastomers are playing an increasingly important role in a diverse range of industrial applications such as automobile components and oil drilling devices.
Core–shell polymers are generally prepared by two methods. The first one is a multistage seeded tandem polymerization, typically carried out in a (micro)emulsion media.17–21 The second one is the self-assembly of the polymerization-induced block copolymer aggregates, which is performed preferably in the polymer solution.22–24 These two methods are most well-known and widely used to prepare the core–shell polymers, which however does not exclude the utilization of other techniques including a hetero-coagulation strategy,25 precipitation polymerization,26 and distillation precipitation polymerization.27 Among these various techniques, multistage seeded emulsion polymerization has been practiced frequently. In this method, polymerization of the shell forming monomer is performed on the surface of the core seeded particles, thus, producing core–shell microspheres. The seeded monomers of the first stages can either be polymerized beforehand in a separate step (so-called “dead” or inactive seeding) or in situ during the emulsion polymerization (so-called “live” or active seeding). The addition of monomers at the first stage and the successive stages can be in a semibatch addition manner (monomer starved feeding) or a batch swelling manner depending on the specific circumstances.
In our previous studies, the uniform spherical NBR nanoparticles28 and PMMA/NBR core–shell nanoparticles (with PMMA and NBR as the core and shell respectively)29 have been synthesized via a GS 12-3-12 emulsified semibatch emulsion polymerization. GS 12-3-12 is the denotation of gemini surfactant trimethylene-1,3-bis(dodecyldimethylammonium bromide) (molar mass 628.69 g mol−1). A gemini surfactant is an amphiphile made up of two conventional surfactant molecules chemically bonded together by a spacer moiety, which features a lower critical micelle concentration (e.g., CMC = 1 mM or ∼0.63 g L−1 for GS 12-3-12) and greatly increased interfacial activity compared to conventional surfactants.30,31
As process-development oriented research and for technical interest, herein we describe the preparation of PMMA/PS/NBR tri-layer core–shell nanoparticles by seeded emulsion polymerization using a three-stage in situ semibatch process by means of which allows scale-up for industrial production of latex particles. In this operational process, all of the monomers including the core monomer MMA were sequentially charged into the reaction system via a dropwise manner (where a monomer starved condition was produced) in an one-pot semibatch reactor. A short term three-stage semibatch emulsion polymerization is used to describe the above process for the facilitation of the following discussions. The prepared unsaturated PMMA/PS/NBR nanoparticles were then directly hydrogenated in latex form in the presence of Wilkinson’s catalyst RhCl(P(C6H5)3)3 to prepare PMMA/PS/HNBR nanoparticles. Latex hydrogenation is a postpolymerization “green” process to directly hydrogenate the unsaturated polymer particles in a stable dispersion (emulsion) preferably in an aqueous medium. The detailed description and fundamental chemistry related to the latex hydrogenation can be found in preceding articles.32,33
Results and discussion
Semibatch emulsion polymerization is a unique process for manufacturing fine polymer nanoparticles. By controlling the monomer feeding rate, the polymer chain growing radicals will consume the monomer molecules faster than the rate of the monomer molecule addition into the reaction system, creating a monomer “starved” condition which is maintained until the end of the polymerization. Therefore, the monomer molecules are considered to be delivered to the reaction locus from an external reservoir through a physical means rather than from the interior of nanodroplets (e.g., microemulsion mechanism). The monomer-starved semibatch microemulsion polymerization provides a practical way to enhance particle formation and thus to produce nanolatex. It has been recently shown that this process can produce a large number of small particles.28,29,34Fig. 1 schematically represents the preparation of PMMA/PS/NBR and PMMA/PS/HNBR tri-layer core–shell nanoparticles. In the reaction process, the PMMA/PS/NBR core–shell nanoparticles are reproducibly prepared using a one-pot three-stage semibatch emulsion polymerization. The first stage monomer MMA, second stage monomer PS, and third stage monomers acrylonitrile (AN) and 1,3-butadiene (BD) (homogenous mixture) were all sequentially charged into one semibatch reaction system under monomer-starved conditions. The PMMA/PS/NBR latex obtained at the end of the reaction was first treated using vacuum distillation to remove the residual BD and most of the unreacted acrylonitrile (AN). The remaining portion of AN was eliminated by steam distillation at 70 °C under reduced pressure since remaining organic chemicals such as AN in the latex may affect the catalytic activity of the catalyst. The catalytic latex hydrogenation was then carried out upon the diene-contained PMMA/PS/NBR nanoparticles using RhCl(P(C6H5)3)3 in the same reactor as used for the synthesis reactions. PMMA/PS/HNBR was finally obtained as the hydrogenated product. The process of this emulsion synthesis can potentially provide additional particle design features such as a modification of the outer or inner surfaces by functional groups. One can also replace the NBR layer with other diene-containing rubbers which allow for subsequent catalytic hydrogenation; for example styrene butadiene rubber (SBR) and polybutadiene rubber (BR).
 | ||
| Fig. 1 Schematic diagram illustrating the synthesis and catalytic latex hydrogenation of diene-containing PMMA/PS/NBR tri-layer core–shell nanoparticles. | ||
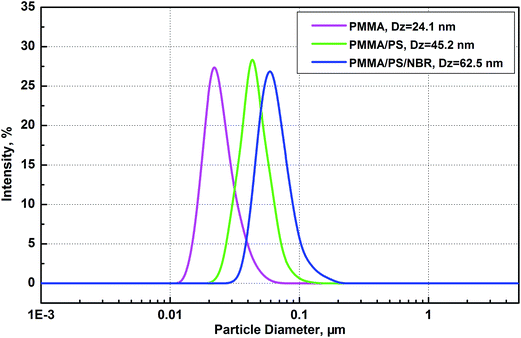
Table 1 provides the principal characteristics of the PMMA, PMMA/PS core–shell, and PMMA/PS/NBR tri-layer core–shell nanoparticles obtained in this semibatch process. The final latex product has a solid content of 21.7 wt% and the NBR outer layer contains a 31 wt% bound level of nitrile. The Dn (number-average diameter) and Dz (intensity-average diameter) of the PMMA/PS/NBR nanoparticles are 51.3 nm and 62.5 nm, respectively. The size of the PMMA, PMMA/PS, and PMMA/PS/NBR nanoparticles was observed to increase appreciably after each stage of polymerization compared to the nanoparticles obtained at the previous stage of polymerization. This pronounced growth indicates that the monomers added during each stage of the reaction were polymerized over the core precursor nanoparticles. TEM imaging also confirms the formation of core–shell structures, as will be discussed later. Fig. 2 shows the particle size distribution (PSD) by intensity for the PMMA, PMMA/PS, and PMMA/PS/NBR nanoparticles, respectively. It can be seen that the all of the latex nanoparticles present a monomodal and narrow distribution of particle size. The PSD can be further quantitatively evaluated by the ratio of Dz/Dn. Dz/Dn values ranging from 1.0–1.1 indicate that the particles are monodispersed while those ranging from 1.1–1.2 are near-monodispersed.29 The dispersity values of particle size presented in Table 1 suggest that near-monodisperse narrow distributions for the PMMA and PMMA/PS nanoparticles can be achieved and the PMMA/PS/NBR nanoparticles have a relatively higher dispersity of 1.22. On the other hand, high molecular weights of PMMA, PMMA/PS, and PMMA/PS/NBR are obtained, e.g., the Mw values are all above 106. The PDIs (Mw/Mn) of these three types of polymers are found to be between 2 and 3, which indicates that the termination of the growing polymer chain mainly occurs by chain transfer to monomer.35 This is consistent with the behavior of chain termination in normal microemulsion polymerization.
| Latex label | First stage | Second stage | Third stage |
|---|---|---|---|
| a Determined via FT-IR analysis and the analysis method can be found in ref. 29.b The solid content (S%) of the polymer emulsion was determined using the weight of dried polymer and polymer latex.c Mn and Mw were determined using GPC and are reported as the number-average molecular weight and weight-average molecular weight, respectively.d Dn and Dz were determined using a DLS technique and are reported as the number average diameter and the intensity average diameter, respectively. | |||
| Particle type | PMMA | PMMA/PS core–shell | PMMA/PS/NBR tri-layer core–shell |
| Temperature (°C) | 70 | 70 | 45 |
| Stirring | Parr stirred reactor | Parr stirred reactor | Parr stirred reactor |
| Turbine type | Four-blade impeller | Four-blade impeller | Four-blade impeller |
| Distance from reactor floor (mm) | 15 | 15 | 15 |
| Speed (rpm) | 200 | 200 | 200 |
| Water (g) | 80 | No additional water | No additional water |
| (NH4)2S2O8 (APS, g) | 0.15 | No additional initiator | No additional initiator |
| GS 12-3-12 (g) | 2.5 | No additional surfactant | No additional surfactant |
| MMA (g) | 4.7 | — | — |
| St (g) | — | 9.1 | — |
| AN and BD mixture (g) | — | — | 13.7 (Homogenous mixture of AN and BD) |
| AN (g) | — | — | 4.1 |
| BD (g) | — | — | 9.6 |
| Monomer feed mode | Semibatch | In situ semibatch | In situ semibatch |
| Feed rate (g min−1) | 0.047 | 0.045 | 0.034 |
| Reaction time after completion of monomer feeding (h) | 1 | 1, Including the time consumed for temperature dropping from 70 to 45 °C | 5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Conversion | |||
| MMA (wt%) | 85.6 | ||
| St (wt%) | 87.2 | ||
| AN (wt%) | — | — | 83.1 |
| BD (wt%) | — | — | 78.4 |
| Bound level of nitrile in NBR shella (wt%) | — | — | 31.0 |
| Solid contentb (wt%) | 4.6 | 12.7 | 21.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Molecular weightc | GPC data | ||
| Mn | 6.5 × 105 | 7.6 × 105 | 6.3 × 105 |
| Mw | 1.7 × 106 | 2.2 × 106 | 1.9 × 106 |
| PDI = Mw/Mn | 2.6 | 2.9 | 3.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Particle diameterd (nm) | DLS data | ||
| Dn (nm) | 20.7 | 38.6 | 51.3 |
| Dz (nm) | 24.1 | 45.2 | 62.5 |
| Dispersity of size (Dz/Dn) | 1.16 | 1.17 | 1.22 |
Fig. 3 shows the appearance of the PMMA (A), PMMA/NBR (B), PMMA/PS (C), PMMA/PS/NBR (D) and PMMA/PS/HNBR (E) nanoparticle latexes. The particle size and solid content of latexes A, C, and D can be found in Table 1. Latex (B) (PMMA/NBR) was reported previously29 and is presented here together with the other latexes for comparison purposes. Latex E is the hydrogenated product of latex D, which has one quarter of the solid content of latex D since a three fold excess of distilled water over the volume of the original latex (v/v) was added during the latex hydrogenation (the experimental method of latex hydrogenation is shown in the ESI†). All of the polymer latexes were coagulated using ethanol. It should be noted that GS 12-3-12 is easily dissolved in both ethanol and distilled water. As shown in Fig. 3, there is a transition in the appearance of latexes A, C and D, varying from transparent to translucent with increasing particle size and solid content. Latex B which is optically transparent, has a number average particle size of 30.6 nm and a solid content of 9.7 wt%. Latex E exhibits a reddish-brown color because of the encapsulation of the catalyst RhCl(P(C6H5)3)3 within the polymer nanoparticles. RhCl(P(C6H5)3)3 is a red colored solid at room temperature. These colloidal dispersions are stable and can remain suspended in water without precipitation for more than one year at around 6 °C inside a refrigerator. This shows that the GS 12-3-12 emulsified semibatch polymerization is an efficient method for synthesizing a stable nanoparticle latex. The zeta potential distribution of the PMMA/PS/NBR nanoparticles is provided in Fig. S2 in the ESI.† The ζ-potential is +46.1 mV at pH = 3.5 and 25 °C, which is in good agreement with the observed colloidal stability of the produced latex. pH = 3.5 is the pH value of the obtained latex since the GS 12-3-12 is an acidic salt (quaternary ammonium bromide) in the aqueous phase. In the present polymerization using a cationic surfactant and (NH4)2S2O8 (APS) as the anionic initiator, a net higher positive ζ-potential of the particles was still measured. This is due to the relatively higher amount of cationic surfactant than APS thereby generating a stronger neutralization effect on the negative ζ-potentials induced by the anionic initiator.
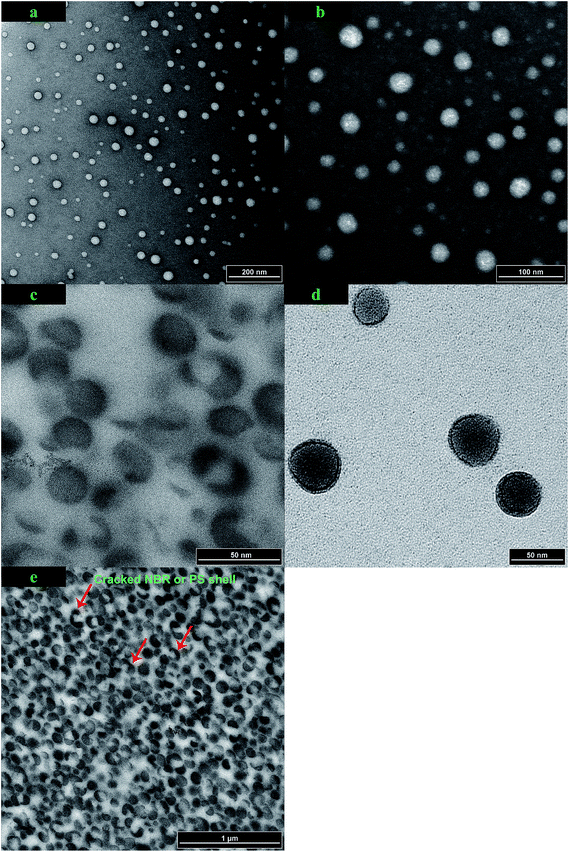
A set of representative TEM images are presented in Fig. 4, which shows the size and morphology of the PMMA nanoparticles, PMMA/PS core–shell nanoparticles, and PMMA/PS/NBR trilayer core–shell nanoparticles. As represented by Fig. 4a, b, and d, the obtained PMMA, PMMA/PS, and PMMA/PS/NBR nanoparticles all exhibit a uniform spherical shape. The dimensions of these three types of nanoparticles determined by TEM are consistent with those measured using a DLS technique (Table 1). However, the distinct core–shell structure could not be directly observed from Fig. 4b because the contrast between the PMMA domain and PS domain is not sufficiently clear. In general, the PS region is darker than the PMMA region in the TEM observation. Therefore, the coverage of the PS layer upon the PMMA polymer weakens the phase contrast between the PMMA and PS phase. The dark background in Fig. 4a and b is due to the existence of the staining agent uranyl acetate on the copper grid, which increases the contrast between the polymer body (white color in these two figures) and the background. In order to verify the formation of the core–shell structure, cross-sectional TEM was carried out as shown in Fig. 4c. It can be seen in Fig. 4c that phase separation between the PMMA core and PS shell was observed due to a grinding operation before sending the samples for analysis by TEM. The PS shell was cracked and bent and the spheres in Fig. 4c are the PMMA domains, which further demonstrates that the PMMA/PS nanoparticles obtained have a core–shell nanostructure (the DLS data has provided prima facie evidence as discussed earlier).
In the TEM characterization in this study, 2% (w/v) uranyl acetate was used to stain the polymer samples. Uranyl acetate is most often used as a negative staining agent, which can enhance the contrast of the sample and the background via a metallic deposit. It is particularly effective for the polymers containing carbon–carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cs). As shown in Fig. 4d, the overall domains of the PMMA/PS/NBR nanoparticles are quite dark and the background regions are relatively light. This contrast in the color is caused by the binding of uranyl acetate to the C
Cs). As shown in Fig. 4d, the overall domains of the PMMA/PS/NBR nanoparticles are quite dark and the background regions are relatively light. This contrast in the color is caused by the binding of uranyl acetate to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cs of the NBR shell. Fig. 4d proves that the NBR has been encapsulated upon the PMMA/PS seeded nanoparticles. The PMMA/PS/NBR nanocomposite was also examined by cross-section TEM as shown in Fig. 4e. In this figure, the NBR shell and the PS shell may coexist and it is difficult to distinguish between the NBR or PS shell. However, a conclusion can be drawn from Fig. 4e that the NBR shell has been formed in the prepared PMMA/PS/NBR nanoparticles.
Cs of the NBR shell. Fig. 4d proves that the NBR has been encapsulated upon the PMMA/PS seeded nanoparticles. The PMMA/PS/NBR nanocomposite was also examined by cross-section TEM as shown in Fig. 4e. In this figure, the NBR shell and the PS shell may coexist and it is difficult to distinguish between the NBR or PS shell. However, a conclusion can be drawn from Fig. 4e that the NBR shell has been formed in the prepared PMMA/PS/NBR nanoparticles.
The control of the particle morphology is an essential part of semibatch emulsion polymerization. There are many formulation and process factors which can influence or control the morphology and structure composition of the resulting nanoparticles, which are characterized by the type and amount of surfactant and initiator, the manner of monomer feeding, the relative hydrophilicity of the core and shell monomers, and the interfacial tensions between the polymer–surfactant interface and the polymer–polymer interface.19,21 The sequential preparation of core and shell particles in multiple stages has been shown here to be an effective approach to obtain complex core–shell structured nanoparticles. The starving induced monomer addition method plays an important role in the formation of a well defined core–shell structure. In this process, the shell monomers have always been at a starving level, which means that the concentration of the shell monomers in the region of each seed is decreased, resulting in that the shell monomers have no time to diffuse into the inner space of the core polymer and hence are driven to polymerize onto the surface of the core nanoparticles to a greater extent. Meanwhile, due to the lower concentration of monomers within the particles, the internal viscosity of the seeded polymerizing particles becomes high, which thus limits the diffusivity of the shell polymer chains and prevents the shell polymer chains from undergoing Ostwald ripening to form separate spherical microdomains in the core region.
The formation of secondary particles was greatly suppressed in the present polymerization system. During the formation of the core–shell nanostructures, the core monomers are first initiated and then polymerized to form numerous precursors, which are able to provide the nuclei sites for the shell monomers to polymerize on. Therefore, the nucleation energy barrier for the shell polymer, which needs to be overcome when no seeds are used, is eliminated, so that the core–shell nanoparticles are formed under kinetic reaction control. The initiation and nucleation of secondary particles is thereby prevented. On the other hand, GS 12-3-12 has a tight packing capability due to its long hydrophobic C12 alkyl chains.36 This constricted packing property results in a cohesive and stable interfacial film around the polymer phase during the polymerization, which enhances the absorption energy between the core polymer domains with surfactant molecules and resists the desorption and repartitioning of the surfactant to stabilize the newly created secondary primary particles.37
RhCl(P(C6H5)3)3, is perhaps the most well-known commercially available catalyst for the hydrogenation of unsaturated rubbers. It can achieve complete hydrogenation of the olefin content (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) without any reduction of the nitrile groups present in NBR. More importantly, it can effectively suppress any crosslinking problems.32
C) without any reduction of the nitrile groups present in NBR. More importantly, it can effectively suppress any crosslinking problems.32
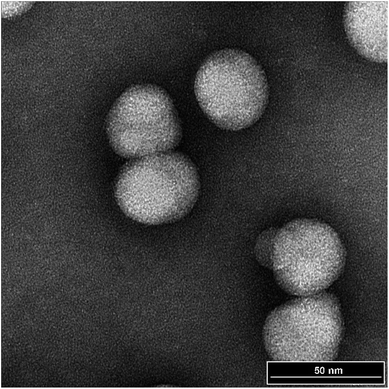
Table 2 presents the operational conditions and hydrogenation results for the direct catalytic hydrogenation of the PMMA/PS/NBR tri-layer core–shell nanoparticles in latex form using RhCl(P(C6H5)3)3. One blank experiment in the absence of catalyst is also listed in Table 2 and was conducted for the purpose of circumventing the influence of the reactor walls and the agitator in the catalytic reactions. It can be seen from Table 2 that the particle size before and after hydrogenation remained almost unchanged on comparing with the original data of the particles in Table 1. The TEM imaging of the hydrogenated PMMA/PS/NBR is also presented in Fig. 5, which confirms that the produced PMMA/PS/HNBR nanoparticles still maintain the spherical morphology even under the high temperature hydrogenation reactions. In addition, comparing Fig. 4d and 5, it can be seen that the PMMA/PS/HNBR appears brighter and less dark than the PMMA/PS/NBR. This can be explained by the considerable reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cs in the HNBR, which results in less binding of uranyl acetate to the PMMA/PS/HNBR phase.
Cs in the HNBR, which results in less binding of uranyl acetate to the PMMA/PS/HNBR phase.
| Latex label | Blank experiment | PMMA/PS/HNBR tri-layer core–shell |
|---|---|---|
| a Determined via 1H NMR analysis (Fig. 6).b Dn and Dz were determined by DLS technique and are reported as the number average diameter and the intensity average diameter, respectively.c Evaluated via a solvent extraction technique.38 | ||
| Temperature (°C) | 130 | 130 |
| H2 pressure (psi) | 1000 | 1000 |
| Stirring | Parr stirred reactors | Parr stirred reactors |
| Turbine type | Four-blade impeller | Four-blade impeller |
| Distance from reactor floor (mm) | 15 | 15 |
| Speed (rpm) | 600 | 600 |
| PMMA/PS/NBR latex (mL) | 25 | 25 |
| Added water to above latex (mL) | 75 | 75 |
| Substrate: NBR outer shell (g) | 3.4 | 3.4 |
| Catalyst: RhCl(P(C6H5)3)3 (g) | — | 0.034 |
| Ligand: TPP (g) | 0.34 | 0.34 |
| RhCl(P(C6H5)3)3/NBR shell (w/w) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| RhCl(P(C6H5)3)3/TPP (w/w) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| Reaction time (h) | — | 3 |
| Degree of hydrogenationa | — | Complete hydrogenation |
| Particle diameter after experimentb (nm) | Based on DLS data | Based on DLS data |
| Dn | 51.8 | 52.1 |
| Dz | 62.9 | 63.4 |
| PDI of size (Dz/Dn) | 1.21 | 1.22 |
| Cross-linkingc | — | Not observed |
A point worthy of attention here is that the latex hydrogenation reaction presented in this work is a “green” process as it is completely free of organic solvent. Fig. 6 shows the 1H NMR spectra of the PMMA/PS/NBR latex nanoparticles both pre- and post-hydrogenation reaction for the reaction catalyzed by RhCl(P(C6H5)3)3 and based on the method presented in Table 2. The broad doublet in the region of 5.0–5.8 ppm is assigned to the olefinic protons due to the existence of the butadiene unsaturated units. The intensity of the broad doublet in the olefinic region decreased gradually during the hydrogenation and almost no resonance at all was observed in this range of 5.0–5.8 ppm after 3 h with a RhCl(P(C6H5)3)3/NBR shell weight ratio of 1 wt% at 130 °C under 1000 psi (6.89 MPa) of H2. This result indicated that complete hydrogenation of the NBR layer was obtained. The PMMA/PS/HNBR tri-layer nanoparticles were thereafter achieved as the hydrogenated product. During the hydrogenation operation, no coagulation of the latex was observed, which indicates that this catalytic hydrogenation has no adverse effect on the latex stability. Crosslinking was evaluated via a solvent extraction technique.38 It was found that no crosslinking occurred in the final hydrogenated product, which demonstrates that the future processibility of the PMMA/PS/HNBR will not be adversely affected by this hydrogenation operation.
In our previous report, we studied the direct hydrogenation of ∼70 nm commercial NBR latex and 97 mol% conversion was reached after 55 h at a temperature of 145 °C under 1000 psi of H2 pressure with a RhCl(P(C6H5)3)3/NBR weight ratio of 1 wt%.39 Under the same level of catalyst loading and lower temperature, the hydrogenation of the PMMA/PS/NBR nanoparticles shows a significantly faster reaction rate (completed hydrogenation within 3 h with 1 wt% RhCl(P(C6H5)3)3/NBR shell at 130 °C under 1000 psi of H2) compared with for the commercial ∼70 nm NBR nanoparticles. This considerable enhancement in the rate of hydrogenation is due to the fact that nanoparticles (Dn = 51.3 nm, Table 1) with a larger specific surface area can increase the capture efficiency for catalyst molecules and the thin layer of less than 20 nm (calculated from the radius difference of PMMA/PS and PMMA/PS/NBR, Table 1) facilitates the diffusion of catalyst molecules inside the NBR chains. The present direct catalytic latex hydrogenation demonstrates that a fast reaction rate can be achieved without using any organic solvent, which may offer exciting avenues in the future for the large-scale production of “green” hydrogenation processes.
These core–shell nanoparticles including PMMA/PS, PMMA/PS/NBR, and PMMA/PS/HNBR are expected to play a unique role as performance-enhancing additives and novel reinforcing agents in high performance rubber compounds. They are uniform, of spherical shape and have a very small particle size with a narrow PSD. These nanoparticles provide an opportunity to achieve a more uniform distribution inside the targeted elastomer, such as the NBR matrix, than in a inorganic filler, such as carbon black and silica, and to meet specific application needs by choosing appropriate core–shell nanoparticles. Given the highly reinforcing nature of these nanoparticles, they can be used as impact modifiers, toughening agents, reinforcing agents, and additives for enhanced performance of the rubber compounds. For example, Wang et al. compared the stress–strain performance of two sulphur vulcanized BR compounds: one reinforced with carbon black and the other with spherical PS/BR nanoparticles with BR as the shell.22 The results showed that the BR reinforced core–shell nanoparticles have a considerably improved modulus at low strains and superior failure properties such as equal tensile strength, higher elongation-to-break, and higher tensile energy-to-break than those using carbon black as the reinforcing filler. As mentioned above, this is because the polymer nanoparticles have a significantly smaller size and are much more easily dispersed in the host rubber than the carbon black fillers, which leads to enhanced compatibility between the host rubber and the reinforcing core of the polymer nanoparticles.
Conclusions
We have demonstrated process-development oriented research which incorporated an emulsion synthesis and “green” catalysis of a new type of unsaturated polymer nanoparticles. PMMA/PS/NBR tri-layer core–shell diene-based nanoparticles were firstly synthesized by a novel three-stage semibatch emulsion polymerization method. The prepared spherical PMMA/PS/NBR nanoparticles have a very thin NBR layer of less than 20 nm and a total z-average diameter of 62.5 nm corresponding to a reasonable solid content of 21.7 wt%. The obtained latex is observed to have a good colloidal stability with a ζ-potential of +46.1 mV at pH = 3.5 and 25 °C. The PMMA/PS/NBR with the NBR outer layer containing 31 wt% nitrile was then used as the substrate for latex hydrogenation in the presence of Wilkinson’s catalyst RhCl(P(C6H5)3)3. A rapid hydrogenation with complete hydrogenation was obtained within 3 h using 1 wt% RhCl(P(C6H5)3)3/NBR shell at 130 °C under 1000 psi of H2. This latex hydrogenation process was carried out in the absence of any organic solvent and no crosslinking was found in the hydrogenated product, the PMMA/PS/HNBR core–shell polymer. This study extends the research on both the synthesis of polymer nanoparticles with complex architecture and the chemical modification of high performance elastomers in the aqueous phase. This may contribute to the development of a “green” process for the commercial hydrogenation of unsaturated rubbers in latex form.Acknowledgements
The authors thank Mr Robert Harris (University of Guelph, Canada) for his assistance with the TEM operation. The authors also gratefully acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC).References
- J. P. Rao and K. E. Geckeler, Prog. Polym. Sci., 2011, 36(887), 887–913 CrossRef CAS PubMed.
- D. R. Paul and L. M. Robeson, Polymer, 2008, 49, 3187–3204 CrossRef CAS PubMed.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- F. Wen, W. Zhang, P. Zheng, X. Zhang, X. Yang, Y. Wang, X. Jiang, G. Wei and L. Shi, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 1192–1202 CrossRef CAS PubMed.
- G. H. Teng and M. D. Soucek, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 4256–4265 CrossRef CAS PubMed.
- K. L. Wooley, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 1397–1407 CrossRef CAS.
- Q. Si, C. Zhou, H. Yang and H. Zhang, Eur. Polym. J., 2007, 43, 3060–3067 CrossRef CAS PubMed.
- J. Zhao, H. Yuan and Z. Pan, J. Appl. Polym. Sci., 1994, 53, 1447–1452 CrossRef CAS PubMed.
- C. Li, D. Wang and C. Liu, J. Dispersion Sci. Technol., 2008, 29, 347–350 CrossRef CAS PubMed.
- S. Wu, Polymer, 1985, 26, 1855–1863 CrossRef CAS.
- A. Wakker, Polymer, 1991, 32, 279–283 CrossRef CAS.
- M. Klapper, S. Nenov, R. Haschick, K. Müller and K. Müllen, Acc. Chem. Res., 2008, 41, 1190–1201 CrossRef CAS PubMed.
- J. W. Taylor and M. A. Winnik, J. Coat. Technol. Res., 2004, 1, 163–190 CrossRef CAS PubMed.
- B. Schuler, R. Baumstark, S. Krisch, A. Pfau, M. Sanor and A. Zosel, Prog. Org. Coat., 2000, 40, 139–150 CrossRef CAS.
- J. Stubbs and D. Sundberg, J. Coat. Technol., 2003, 75, 59–67 CrossRef CAS.
- D. Basset, J. Coat. Technol., 2001, 73, 42–55 CrossRef.
- C. F. Lee, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 2224–2236 CrossRef CAS PubMed.
- M. Zhang, Y. Lan, D. Wang, R. Yan, S. Wang, L. Yang and W. Zhang, Macromolecules, 2011, 44, 842–847 CrossRef CAS.
- W. J. Liu, W. D. He, Y. M. Wang, D. Wang and Z. C. Zhang, Polym. Int., 2006, 55, 520–524 CrossRef CAS PubMed.
- C. J. Ferguson, G. T. Russell and R. G. Gilbert, Polymer, 2003, 44, 2607–2619 CrossRef CAS.
- S. Tolue, M. R. Moghbeli and S. M. Ghafelebashi, Eur. Polym. J., 2009, 45, 714–720 CrossRef CAS PubMed.
- X. R. Wang, J. E. Hall, S. Warren, J. Krom, J. M. Magistrelli, M. Rackaitis and G. G. A. Bohm, Macromolecules, 2007, 40, 499–508 CrossRef CAS.
- C. Garcia, Y. Zhang, S. Mahajan, F. DiSalvo and U. Wiesner, J. Am. Chem. Soc., 2003, 125, 13310–13311 CrossRef CAS PubMed.
- M. Q. Chen, T. Serizawa, A. Kishida and M. Akashi, J.Polym. Sci., Part A: Polym. Chem., 1999, 37, 2155–2166 CrossRef CAS.
- K. Sanguansap, T. Suteewong, P. Saendee, U. Buranabunya and P. Tangboriboonrat, Polymer, 2005, 46, 1373–1378 CrossRef CAS PubMed.
- W. H. Li and H. D. H. Stover, Macromolecules, 2000, 33, 4354–4360 CrossRef CAS.
- F. Bai, X. L. Yang and W. Q. Huang, Macromolecules, 2004, 37, 9746–9752 CrossRef CAS.
- H. Wang, Q. Pan and G. L. Rempel, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4656–4665 CrossRef CAS PubMed.
- H. Wang, M. Hammond and G. L. Rempel, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 736–749 CrossRef CAS PubMed.
- R. Zana, M. Benrraou and R. Rueff, Langmuir, 1991, 7, 1072–1075 CrossRef CAS.
- F. M. Menger and J. S. Keiper, Angew. Chem., Int. Ed., 2000, 39, 1906–1920 CrossRef.
- H. Wang, L. Yang and G. L. Rempel, Polym. Rev., 2013, 53, 192–239 CrossRef CAS PubMed.
- H. Wang, L. Yang, S. Scott and G. L. Rempel, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4612–4627 CrossRef CAS PubMed.
- S. Sajjadi, Langmuir, 2007, 23, 1018–1024 CrossRef CAS PubMed.
- A. P. Full, E. W. Kaler, J. Arellano and J. E. Puig, Macromolecules, 1996, 29, 2764–2775 CrossRef CAS.
- S. K. Hait and S. P. Moulik, Curr. Sci., 2002, 82, 1101–1111 CAS.
- J. W. Ha, I. J. Park, S. B. Lee and D. K. Kim, Macromolecules, 2002, 35, 6811–6818 CrossRef CAS.
- (a) Y. He, E. S. Daniels, A. Klein and M. S. El-Aasser, J. Appl. Polym. Sci., 1996, 65, 511–523 CrossRef; (b) Y. He, E. S. Daniels, A. Klein and M. S. El-Aasser, J. Appl. Polym. Sci., 1997, 64, 1143–1152 CrossRef CAS.
- Z. Wei, J. Wu, Q. Pan and G. L. Rempel, Macromol. Rapid Commun., 2005, 26, 1768–1772 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03957f |
| This journal is © The Royal Society of Chemistry 2015 |