Fabrication of lanthanum-based phosphate binder using cross-linked alginate as a carrier
Abstract
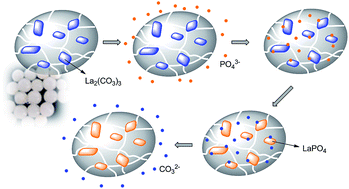
In this paper, phosphate binding properties of lanthanum carbonate loaded sodium alginate cross-linked beads (LaC@SA) were investigated. The beads were shaped when the alginate solution (with La2(CO3)3 dispersed in it) was dropped in calcium ion solution. During this process, the alginate macromolecular chains combined with each other. The morphology of the LaC@SA beads was observed using scanning electron microscopy. Thermal gravimetric analysis and X-ray diffraction analysis were also applied to confirm the existence of the LaC solid in the beads. The LaC@SA beads showed good swelling abilities in different aqueous circumstances. The phosphate binding behavior was investigated through the molybdenum blue spectrophotometric method. The beads exhibited maximum phosphate binding capacity at pH 5 and 45 °C, but at the same time, the capacity showed no remarkable differences from 25 to 65 °C or pH from 1 to 7. Thus, the LaC@SA beads may have potential applications in environmental management, wastewater treatment and moreover, treatment of hyperphosphatemia patients.

 Please wait while we load your content...
Please wait while we load your content...