Room temperature deep eutectic solvents of (1S)-(+)-10-camphorsulfonic acid and sulfobetaines: hydrogen bond-based mixtures with low ionicity and structure-dependent toxicity†
Fabio Cardellinia,
Raimondo Germani*a,
Gianluigi Cardinaliab,
Laura Corteb,
Luca Roscinib,
Nicoletta Spretic and
Matteo Tieccoab
aCEMIN, Centre of Excellence on Nanostructured Innovative Materials, Department of Chemistry, Biology and Biotechnology, University of Perugia, via Elce di Sotto 8, I-06123 Perugia, Italy. E-mail: raimondo.germani@unipg.it
bDepartment of Pharmaceutical Sciences – Microbiology, University of Perugia, via Borgo XX giugno 74, I-06121, Perugia, Italy
cDepartment of Physical and Chemical Sciences, University of L'Aquila, via Vetoio, Coppito, I-67100, L'Aquila, Italy
First published on 27th March 2015
Abstract
Twelve novel deep eutectic solvents (DESs) were prepared and characterized in this work. They are mixtures of (1S)-(+)-10-camphorsulfonic acid (CSA) and differently structured sulfobetaines (SBs) with aliphatic, aromatic and amphiphilic moieties. They are liquids at room temperature, their melting points span, in fact, from −5° to 19 °C, so we can name these mixtures RTDESs (room temperature deep eutectic solvents). These zwitterionic DESs were characterized in terms of their viscosity, conductivity (and therefore ionicity via Walden plots), density, surface tension and toxicity on eukaryotic model cells. The collected data suggest that the interaction between CSA and the SBs can be ascribed as a hydrogen bond instead of a proton transfer, therefore they are not ionic liquids. To our knowledge, their position on the Walden plot, in the left portion close to the diagonal, has not yet been observed for other DESs or ionic liquid systems and indicates the low ionicity of these mixtures. A FTIR-based bioassay was performed to determine the toxicity of these mixtures on eukaryotic model cells (Saccharomyces cerevisiae). The DESs showed merely a dehydrating effect on the cells, similar to that produced by CaCl2, a low cell toxicity salt. This supports these DESs as promising green media. Amphiphilic SBs DESs showed a stronger effect on the cells and a structure-activity trend can be described for this class. A preliminary study on the use of these novel DESs as Brønsted catalyst media was accomplished by the use of one of them in chalcone synthesis, which showed promising catalytic and recycling capabilities.
Introduction
The development of new reaction media plays a key-role within the green chemistry framework. The goal of obtaining novel media with low environmental impact represents, in fact, a challenge in modern chemistry, considering the toxicity and the high vapour pressure of traditional organic solvents.1–3 Ionic liquids (ILs) are surely the most studied and developed solvents in this field in the last decades. ILs are formed by organic cations and organic or inorganic anions, and they are liquid at room temperature or at temperatures under 100 °C.4 ILs have important physico-chemical advantages in terms of “greenness” compared to typical organic solvents, a non-exhaustive list of which includes: low vapour pressure, high thermal stability and non-flammability. Moreover, they have broad liquid ranges and very high solubilizing capabilities.5,6 Unfortunately, ILs were found to be expensive and toxic.7–10For these reasons bio-based mixtures such as Bio-ILs11 and Bio-based solvents12 have gained relevance in literature more recently. The Bio-based solvents class includes all the mixtures with natural and biomass-derived substances, e.g. glycerol,13 lactic acid,14 γ-valerolactone,15 limonene,16 and water solutions of carbohydrates.17,18
Among these systems, deep eutectic solvents (DESs) represent a novel promising class of green solvents.19 DESs are a family of solvents generally liquid at temperatures lower than 100 °C. They can be prepared by simply mixing high-melting point onium salts, such as ammonium or phosphonium, with neutral compounds. The hydrogen-bond interaction that occurs within the donor (HBD) and the acceptor (HBA) molecules determines a considerable reduction of the melting point of the mixtures, therefore a liquid system formation.20,21 For their simplicity of preparation, the atom economy, and the low cost of many starting products, DESs have gained particularly attention as substitutes of conventional volatile organic solvents in many chemical processes. DESs have found multiple applications in chemistry and in different technological applications such as: electrochemistry,22–25 drug delivery,26 organic synthesis,27–31 solubilization32–34 and bioconversion.35–37
The binary system choline chloride (ChCl)–urea was the first DES developed, and it is the most widely studied and used.38–42 Other HBD compounds are able to form DESs in mixture with ChCl such as phenols,43 carboxylic acids,44 glycerol,45 sugars,46,47 and alcohols.48 In the cases of mixtures of natural compounds, such as amino acids, sugar and carboxylic acids, these systems are named Natural Deep Eutectic Solvents (NADES)49 because derived from natural resources.
The interaction of the anion of the salt, which is usually a chloride, with the HBD molecule is the driving force that leads to the reduction of the melting points of the mixtures, therefore on liquid systems. Halides can be nucleophilic and oxidizing agents, and they can lead to unwanted by-products such as halogenidric acids, which are also dangerous. For these reasons a halogen-free DES is strongly desirable. For this purpose zwitterionic systems such as betaines are interesting molecules, because they do not have a counterion that can impact on DES properties. Moreover betaines are cheap and have low toxicity.50,51
Nowadays, there are few examples of deep eutectic solvents that are based on zwitterionic systems (carboxy- or sulfobetaines). A series of DESs obtainable from glycine betaine with different neutral hydrogen bond donor compounds has recently been reported.52 In our previous paper we have shown how carboxybetaine trimethylglicine is able to form DESs when mixed with carboxylic acids.53 The depression of the melting point was established by the onset of the hydrogen bond between the trimethylglicine carboxylate with the acid hydrogen of the carboxylic group. These DESs are liquid at temperatures lower than 70 °C, they present a good viscosity and conductivity values, they derive from bio-renewable resources and above all they are not toxic.
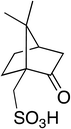
This work is a continuation of our previous investigations to find novel, different and tailor-made deep eutectic solvents, without halides ions, with Brønsted acid properties, to use as solvents and catalysts.53–56 The focus of this study reported, was to develop a new class of DESs using an organic solid sulfonic acid as HBD compound and zwitterionic sulfobetaine molecules as HBA. As sulfonic acid, commercially available (1S)-(+)-10-camphorsulfonic acid (CSA) was chosen, because of its high melting point (198 °C) and because of the absence of hydration water that can generate solutions instead of true eutectic systems (Fig. 1).
Liquid mixtures of sulfobetaines compounds with strong acids, such as sulfuric acid, are reported in literature.57–60 These systems were obtained by mixing water solutions of these molecules, then removing water by evaporation under vacuum. The complete removal of water from ILs formed with mineral acids (e.g. H2SO4) can be very hard to obtain. Moreover, the small difference of pKa values between the two sulfonic groups (sulfobetaines pKa ≈ −2; sulfuric acid pKa1 = −3, pKa2 = 1.9) suggests that the proton cannot be completely transferred from one molecule to another, but indicates a more probable hydrogen bonding interaction formation.
In this work we demonstrate that the interaction between sulfobetaines and a sulfonic acid leads to a deep eutectic solvent instead of IL. This is due to similar pKa values of CSA and sulfobetaines (e.g. ArSO3H pKa = −2.5; PTSA pKa = −2.8; MsOH pKa = −1.9; CSA pKa = 1.2). We prepared sundry DESs from differently structured sulfobetaines: aliphatic, aromatic and amphiphilic sulfobetaines with propyl or butyl spacer were synthesized. These DESs were characterized in terms of their conductivity, viscosity, ionicity (via Walden plot), density, and surface tension. Their toxicity on eukaryotic model cells, such as Saccharomyces cerevisiae yeast, was determined with a FTIR-based bioassay we developed in the recent years.61–63 The advantage of the use of these DESs as reaction media and catalysts was valued with a Claisen–Schmidt reaction performed in one of the novel DESs.
Experimental
Materials
Solvents of analytical grade, (1S)-(+)-10-camphorsulfonic acid 99%, 1,3-propanesultone, 1,4-butanesultone, N,N-dimethyl-butylamine, tributylamine, N,N-dimethyl-cyclohexylamine, N-methyl-N-octyloctanamine, 1-methyl-imidazole, 1-butyl-imidazole, pyridine, N,N-dimethyl-tetradecylamine, N,N-diethyl-tetradecylamine, N,N-dipropyl-tetradecylamine, benzaldehyde, acetophenone and surfactants sulfobetaines N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (SB3-12) and N-tetradecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (SB3-14) were purchased from Sigma-Aldrich, Fluka and Alfa Aesar. 1-methyl-imidazole, 1-butyl-imidazole and N-methyl-N-octyloctanamine were distilled prior to use. Surfactant sulfobetaines SB3-12 and SB3-14 were purified by double recrystallization from acetone–methanol mixture prior to use.Synthesis of sulfobetaines
The sulfobetaines were synthesized from 1,3-propanesultone (1,4-butanesultone for SB4-14) by reaction with the appropriate tertiary amine to reflux in acetonitrile or toluene for 2–10 h. All the molecules were synthesized according to previous procedures reported in literature and their recorded melting points and 1H-NMR spectra agree with the same literature data. 1H-NMR spectra were measured at 25 °C with a Bruker 200 MHz instrument in D2O or CDCl3 solutions with tetramethylsilane (Me4Si) as internal standard. Melting points were obtained on a Barloworld Scientific Stewart SMP3 micro melting point apparatus and are uncorrected. Agilent 6850 Series II Network Gas Chromatography instrument (column DB-35MS l = 30 m, d = 0.32 mm, film = 0.25 mm) was used for GC analysis. Values of the critical micelle concentration (c.m.c.) were determined from plots of surface tension vs. – log [surfactant]. No minima could be observed in these plots. Surface tensions were measured on a Fischer, du Nouy type tensiometer.The characterizations of all the molecules are reported here: SBE3-14:64 Yield 80%, m.p. 134–135 °C, 1H-NMR (200 MHz CDCl3) δ = 3.59–3.72 (m, 2H, N+CH2), 3.28–3.46 (m, 4H, N+(CH2CH3)2), 3.05–3.20 (m, 2H, RCH2N+), 2.92 (t, 2H, CH2SO3−), 2.09–2.28 (m, 2H, N+CCH2C), 1.58–1.78 (m, 2H, RCH2CN+), 1.38 (t, 6H, 2 CH3), 1.20–1.39 (m, 22H, 11 CH2), 0.89 (t, 3H, CH3), c.m.c. = 2.55 × 10−4 M; SBP3-14:64 Yield 75%, m.p. 124–125 °C, 1H-NMR (200 MHz CDCl3) δ = 3.55–3.66 (m, 2H, N+CH2), 3.10–3.30 (m, 6H, (CH2)3N+), 2.92 (t, 2H, CH2SO3−), 2.10–2.30 (m, 2H, N+CCH2C), 1.58–1.89 (m, 6H, (CH2C)3N+), 1.20–1.40 (m, 22H, 11 CH2), 1.04 (t, 6H, 2 CH3), 0.89 (t, 3H, CH3), c.m.c. = 1.99 × 10−4 M; SB3-8 bis:64 Yield 94%, m.p. 157–158 °C, 1H-NMR (200 MHz, CDCl3) δ = 0.89 (t, 6H, 2CH3); 1.29–1.62 (m, 24H, 12CH2); 2.20 (m, 2H, N–C–CH2–C–SO3); 2.90 (m, 2H, –CH2–SO3); 3.11 (s, 3H, CH3); 3.22 (m, 4H, (R–CH2)2N–); 3.51–3.66 (m, 2H, –N–CH2–C–C–SO3); SB3-MIM:65 Yield 98%, m.p. 218–219 °C, 1H-NMR (200 MHz D2O) δ = 9–7 (s + d, 3H, Ar); 4.1–4.2 (m, 2H, CH2N+); 3.7 (s, 3H, CH3N); 2.7–2.8 (t, 2H, CH2SO3−); 2.0–2.1 (m, 2H, CH2CH2SO3−); SB3-BIM:66 Yield 95%, m.p. 176–177 °C, 1H-NMR (200 MHz, D2O) δ = 9–7 (m, 3H, Ar); 4–4.1 (dt, 4H, (CH2)2N+); 2.7–2.8 (t, 2H, CH2SO3−); 2.1 (m, 2H, CH2CH2SO3−); 1.6–1.7 (m, 2H, CH2CH2CH3); 1.1–1.2 (m, 2H, CH2CH3); 0.7–0.8 (t, 3H, CH3); SB3-Cy:67 Yield 98%, m.p. 115–116 °C, 1H-NMR (200 MHz, D2O) δ = 3.2–3.5 (m, 3H. CH2N+ + CHN+); 2.7–2.8 (m, 8H, (CH3)2N+ + CH2SO3−); 2.0 (m, 2H, CH2CH2SO3−); 1.1–1.8 (m, 10H, CH2Cy); SB3-Py:68 Yield 97%, m.p. 277–278 °C, 1H-NMR (200 MHz D2O) δ = 9–7 (m, 5H, Ar); 4.5 (m, 2H, CH2N+); 2.8–2.9 (t, 2H, CH2SO3−); (m, 2H, CH2CH2SO3−); SB4-14:51 Yield 85%, m.p. 273–274 °C, 1H-NMR (200 MHz CDCl3) δ = 3.6 (m, 2H, N+CH2RSO3−), 3.1–3.3 (m, 8H, N+(CH3)2 + N+CH2C13), 2.9 (t, 2H, CH2SO3−), 1.9 (m, 4H, CH2CH2CH2SO3−), 1.6–1.8 (m, 6H, N+CH2CH2C12 + N+(CH2CH2CH3)2), 1.3 (m, 22H, (CH2)11CH3), 0.85 (t, 3H, (CH2)13CH3), c.m.c. = 2.80 × 10−4 M; SB3-4:64 Yield 98%, m.p. 270–271 °C, 1H-NMR (200 MHz, D2O) δ = 3.1–3.3 (m, 4H, (CH2)2N+); 2.9 (s, 6H, (CH3)2N+); 2.7–2.8 (t, 2H, CH2SO3−); 2.0–2.1 (m, 2H, CH2CH2SO3−); 1.5–1.6 (m, 2H, CH2CH2CH3); 1.1–1.2 (m, 2H, CH2CH3); 0.8 (t, 3H, CH3); SB3-4 Tris:69 Yield 96%, m.p. 219–220 °C, 1H-NMR (200 MHz, D2O) δ = 3.0–3.2 (m, 6H (CH2)3N+); 2.7–2.8 (t, 2H, CH2SO3−); 1.9–2.9 (m, 2H, CH2CH2SO3−); 1.5–1.6 (m, 6H (CH2CH2CH3)3); 1.1–1.3 (m, 6H, (CH2CH3)3); 0.7 (t, 6H, CH3).
DESs preparation
(1S)-(+)-10-Camphorsulfonic acid (CSA) was dried under vacuum over P2O5 prior to use. The sulfobetaine and CSA, at proper molar ratio, were directly weighed in a flask fitted with a stopper. The solid mixture was magnetically stirred and heated at 90 °C until a liquid was formed (typically 20–30 minutes).DESs melting points determination
In a 10 mL one-necked, round-bottomed flask, equipped with a thermometer used as stopper, weighed amounts of sulfobetaine and (1S)-(+)-10-camphorsulfonic acid were introduced. The binary systems were heated until they became homogenous liquids, and then were gradually cooled first to room temperature, then immersed in a water–ice bath (or ice–NaCl mixture for lower temperatures) for melting point determination.Viscosity measurements
The viscosity of the eutectic mixtures was measured by using a Fungilab Expert L viscometer, fitted with a thermostatic jacket and a temperature probe. The viscometer jacket was connected to an external thermostated bath. The viscosity measurements were obtained using a spindle attachment. Readings were taken after 20–25 minutes for each temperature.Conductivity measurements
Conductivity measurements were performed on an Analytical Control conductometer (model 120) equipped with a platinum cell (cell constant = 1.05 cm−1). Sample temperature was maintained constant by a glass-jacked beaker connected to a thermostatic bath. To ensure correlation between the results, the same sample was used for conductivity and viscosity experiments. The readings were taken after 20–25 minutes for each selected temperature.Density measurements
The density values at eutectic ratio at different temperatures were obtained by measuring the weight of the sample in a 2.00 mL volumetric flask. The flasks were held in a thermostated bath for 1 hour then brought to volume by eliminating the amount of liquid with a Pasteur pipette. The flask was then thermostated at room temperature for 1 hour and then weighed on analytical balance.Surface tension measurements
Surface tension of eutectic mixtures was measured at 25 °C using a Sigma 700 Force Tensiometer with a platinum ring (diameter 1.9 cm).FTIR bioassay measures
In order to test the activity of the compounds used in this study, the yeast strain Saccharomyces cerevisiae CMC 520 was employed as target. It was obtained from the internal collection of the Microbial Genetics and Phylogenetics Laboratory of the Department of Pharmaceutical Sciences (University of Perugia) and it is also deposited in the collection of the Centraalbureau voor Schimmelcultures (CBS) as CBS 13873. The D1/D2 domain of the LSU (26S) gene showed that this strain is 99.8% similar to the species type strain, thus allowing to identify it as an authentic S. cerevisiae strain70 (data not shown).Pre-culture was inoculated at OD600 = 0.2 in 200 mL of YEPD + chloramphenicol medium (Yeast Extract 1%, Peptone 1%, Dextrose 2% – chloramphenicol 0.5 g L−1 – Difco Laboratories, Detroit, MI, USA) and grown 18 h at 25 °C, under shaking at 150 rpm. Cell suspension was centrifuged at 4143g (4500 rpm) for 3 min, resuspended in distilled sterile water, divided in fourteen 50 mL tubes, washed twice with distilled sterile water and pelleted. A 2 g weight for each DES and for anhydrous CaCl2 (Sigma Aldrich), was sampled in thirteen 50 mL tubes and the previously prepared cell pellets were added to these tubes. The control consisted of pelleted cells. The same experiment was conducted with CaCl2·2H2O, leading to the same results (data not shown).
The tubes were incubated 2 min at 25 °C, then 5 mL of distilled sterile water were added in each tube, centrifuged 3 min at 4143g, washed twice with distilled sterile water and re-suspended in 1.5 mL HPLC grade water in polypropylene tubes. A 105 μl volume was sampled for three independent FTIR readings (35 μl each, according to the technique suggested by Manfait and co-workers71), while 100 μl were serial diluted to determine the viable cell counting of tests and control suspension, in triplicate, on YEPDA plates. The biocidal effect of the compounds was tested as cell mortality induced by these compounds. The cell mortality (M) was calculated as M = [(1 − Cv)/Ct] × 100.
The FTIR experiments were carried out with a TENSOR 27 FTIR spectrometer, equipped with HTS-XT accessory for rapid automation of the analysis (BRUKER Optics GmbH, Ettlingen, Germany).
FTIR measurements were performed in transmission mode. All spectra were recorded in the range between 4000 and 400 cm−1. Spectral resolution was set at 4 cm−1, sampling 256 scans per sample. The software OPUS version 6.5 (BRUKER Optics GmbH, Ettlingen, Germany) was used to carry out the quality test, baseline correction, vector normalization and the calculation of the first and second derivatives of spectral values.
IR data analyses
The script MSA (Metabolomic Spectral Analysis) employed in this study was developed in “R” language to carry out the statistical analysis on the matrices of spectral data exported as ASCI text from OPUS 6.5. The analytical procedure consisted in normalizing raw spectra as suggested by Goodacre and coll.,72 calculating the Response Spectra (RS) as described by Corte and colleagues61 and finally by calculating the correlations between normalized spectra and between RS. RS are defined as the difference between the average spectrum of the cells challenged by the tested compound and that of the cells resting in water for the same time. In order to highlight the differences exerted not only by single compounds but also by their class of appurtenance (i.e. amphiphilic and non-amphiphilic SBs DESs), the single RSs of the members of the same class were averaged producing the Average Response Spectra (ARS). Amphiphilic SBs–CSA mixtures: SB3-14, SBE3-14, SBP3-14, SB3-12, SB4-14, SB3-8 bis–CSA. Aromatic–aliphatic SBs–CSA mixtures: SB3-MIM, SB3-MIM, SB3-4, SB3-4 Tris, SB3-Cy, SB3-Py–CSA. The spectral regions (W1, W2, W3, W4, W5) are reported as suggested by Naumann and colleagues.73Chalcone synthesis procedure
0.33 mmol of benzaldehyde and 0.33 mmol of acetophenone were added to SB3-Cy–CSA DES (1 mmol CSA, 0.7 mmol SB3-Cy) under magnetic stirring. The reaction was conducted at 90 °C for 16 h. The mixture was then cooled to room temperature and then transferred into a separator funnel and washed five times with ethyl acetate–H2O. The organic phase was dried under Na2SO4 and then filtered. The solvent was evaporated under vacuum to give a orange solid. The product was purified via chromatographic column with H2O–EtOH 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mixture as eluent. A pale yellow solid was obtained. Yield 70%, m.p. 56–58 °C; 1H-NMR (200 MHz, CDCl3) δ = 8.0 (d, 2H, H-β + H-4′); 7.8 (d, 1H, H-α); 7.6 (d, 2H, H-2′, H-6′); 7.5 (dt, 4H, H-2, H-6, H-3′, H-5′); 7.4 (m, 3H, H-3, H-4, H-5).
20 mixture as eluent. A pale yellow solid was obtained. Yield 70%, m.p. 56–58 °C; 1H-NMR (200 MHz, CDCl3) δ = 8.0 (d, 2H, H-β + H-4′); 7.8 (d, 1H, H-α); 7.6 (d, 2H, H-2′, H-6′); 7.5 (dt, 4H, H-2, H-6, H-3′, H-5′); 7.4 (m, 3H, H-3, H-4, H-5).
The conversion was determined in a separate experiment via addition to the reaction mixture of a specific amount (approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of molar ratio with reagents) of tert-butylbenzene (Sigma-Aldrich, 99%) as internal standard after 16 h. The conversion was determined via gas chromatography analyses.
1 of molar ratio with reagents) of tert-butylbenzene (Sigma-Aldrich, 99%) as internal standard after 16 h. The conversion was determined via gas chromatography analyses.
Results and discussion
DESs preparation
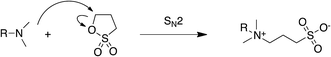
The synthesis of several sulfobetaines was the first step in DESs preparation. The HBA molecules were synthesized via reaction of tertiary amines with 1,3-propanesultone. The reaction scheme is reported in Fig. 2.The reaction is a SN2 substitution, which for ring opening leads directly to the formation of the sulfobetaine without the production of any side-product salt. Twelve differently structured sulfobetaines were synthesized, with aliphatic, aromatic and amphiphilic moiety (Table 1). 1,4-Butanesultone was used for the synthesis of 4-(tetradecyldimethylammonio)butan-1-sulfonate, which has a tetramethylene spacer between the ammonium and the sulfonate groups.
| Entry | Sulfobetaine structure | Acronym | DES eutectic ratio | SB m.p. (°C) | DES m.p. (°C) | Δt (°C) |
|---|---|---|---|---|---|---|
| a m.p. of CSA = 196 °C. | ||||||
| 1 | 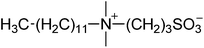 |
SB3-12 | 1.5 | 259 | −1 | 260 |
| 2 | 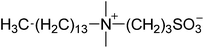 |
SB3-14 | 1.5 | 256 | −3 | 259 |
| 3 | 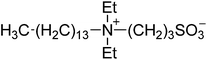 |
SBE3-14 | 1.5 | 134 | 4 | 130 |
| 4 | 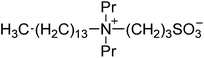 |
SBP3-14 | 1.5 | 124 | 3 | 121 |
| 5 | 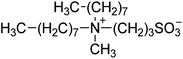 |
SB3-8 bis | 1.5 | 157 | −5 | 162 |
| 6 | 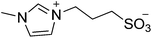 |
SB3-MIM | 1.5 | 219 | −4 | 223 |
| 7 | 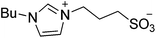 |
SB3-BIM | 1.5 | 177 | 0 | 177 |
| 8 | 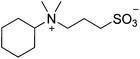 |
SB3-Cy | 1.5 | 116 | 9 | 107 |
| 9 | 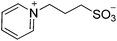 |
SB3-Py | 1.5 | 278 | 19 | 259 |
| 10 | 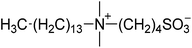 |
SB4-14 | 2.0 | 273 | −5 | 278 |
| 11 | 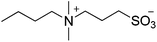 |
SB3-4 | 2.0 | 270 | 13 | 257 |
| 12 | 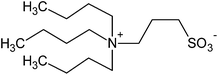 |
SB3-4 Tris | 2.0 | 219 | 9 | 210 |
The second step in DESs preparation was eutectic melting point determination of SBs–CSA mixtures. The mixtures were prepared by simply mixing the compounds at 90 °C, and resulted amber liquids for all the twelve betaines at this temperature. The melting points of the DESs were determined by varying the molar fraction (x) of the (1S)-(+)-10-Camphorsulfonic acid. In Fig. 3 are reported the profiles of melting point vs. molar fraction x of SB3-Py–CSA and SB3-14–CSA mixtures (see ESI† section for all the profiles).
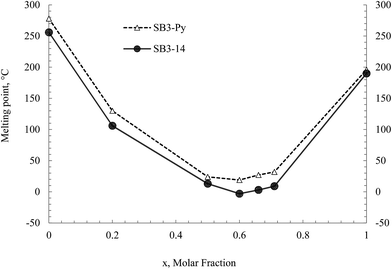 | ||
| Fig. 3 Melting points of SB3-Py and SB3-14 with CSA as a function of acid molar fraction x. Legend (hollow triangles) SB3-Py–CSA mixture, (solid circles) SB3-14–CSA mixture. | ||
A lowering of the melting point (compared to the ones of CSA and of the SB) was observed for 0.2 molar fraction of CSA–SB mixtures for all the betaines. A further stronger decrease was observed for 0.5 molar fraction. Little variations were observed for subsequent additions of acid until 0.7 molar fraction.
In Table 1 are reported: the molar eutectic ratios of the DESs, the melting points of the sulfobetaines (SBs), the melting points of the DESs and the Δt of melting point between the SBs and the DESs observed for all our twelve mixtures.
All the mixtures showed a eutectic ratio of 1.5, while SB4-14, SB3-4 and SB3-4 Tris–CSA mixtures (entry 10, 11, 12) showed a 2.0 molar eutectic ratio value. For SB3-4 and SB3-4 Tris–CSA mixtures, these values could be due to the small dimensions and to the symmetry of the sulfobetaines. These can impact on their packing capability, so they need a higher amount of CSA to form a DES. SB4-14 sulfobetaine has a longer spacer and it can provoke a folding of the chain that determines an increasing of interactions between the positive and the negative charges so a higher amount of CSA is needed to form the DES. This partial neutralization of the charges has also impact on the solubility in water of SB4-14 sulfobetaine (0.022 M) that is much lower compared to SB3-14 molecule (0.92 M).64
There is a strong depression of the melting point of the mixtures (Δt from 107 to 273 °C) in all the realized systems: this is indicative of strong interactions between the SB and the CSA.19 Moreover the decrease of entropy, therefore a lower Δt, is generally related to the structure of the crystal lattice of solid SB. The greater the symmetry of the lattice is, the greater the decreasing of Δt is provoked.
In the SBR3-14–CSA mixtures series (entry 2, 3, 4) an increase of steric hindrance of alkyl residues (R = Me, Et, n-Pr) on ammonium led to a decrease of Δt. The increase of the bulkiness of the ammonium group of sulfobetaines leads, in fact, to a lower interaction between the positive and the negative charges. In this case the coulomb interaction is lower and the related entropic gain is smaller. This effect is relevant also for SB4-14–CSA mixture (entry 10) where a higher Δt decrease is present: the opposite charge vicinity provokes a higher increase of entropy gain. In SB3-8 Bis, SB3-12 and SB3-14 mixtures a decrease in chain length led to a reduction of Δt. SB3-8 Bis–CSA mixture showed a smaller Δt than SB3-12–CSA (entry 1, 5) because it has a lower symmetry of the SB, due the two bulky octyl chains. The same reasons can be considered for SB3-MIM and SB3-BIM mixtures (entry 6, 7). The butyl chain caused lower symmetry in the sulfobetaine lattice of SB3-BIM and lower entropy change in presence of CSA. SB3-4–CSA system have lower symmetry than SB3-4 Tris–CSA mixture (entry 11, 12) but the three butyl chains are very bulky and lead to a more difficult packing. SB3–Cy mixture (entry 8) presented a lower value of Δt than the linear alkyl SB realized in this work, because of the lower van der Waals interactions in the cyclohexyl group therefore lower lattice symmetry. SB3-Py–CSA mixture (entry 9) led to a great Δt value, due to the π–π interactions in SB structure. This DES has the highest melting point in our set (19 °C) but it is close to the melting points of the other DESs.
The melting points of the DESs are quite similar for all the mixtures realized in this work, and they span between −5 °C to 19 °C, so we can name these mixtures RTDESs (room temperature deep eutectic solvents). This could indicate that the driving forces that determine the liquid formation are the same for all the DESs prepared, because they involve the same portions of the molecules for all the mixtures.
Viscosity
The viscosity of the DESs is exponentially dependent on the temperature following the Arrhenius equation (eqn (1)).
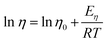 | (1) |
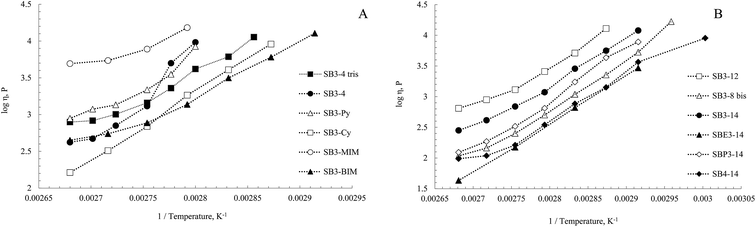
Viscosity is related to the radius of the vacancies in the structures compared to the radius of the molecules.74,75 Natural logarithm of η vs. 1/T profiles of our DESs are reported in Fig. 4. The DESs from aliphatic and aromatic SB are reported in panel A, the ones from amphiphilic SB in panel B. The viscosities (η) and viscosity activation energies (Eη) measured for our DESs are reported in Table 2.
| Entry | SB | ηa (cP) | Eηb (kJ mol−1) |
|---|---|---|---|
| a Values obtained at 85 °C.b Energy of activation extrapolated at 25 °C. | |||
| 1 | SB3-12 | 3025 | −38.2 |
| 2 | SB3-14 | 2161 | −41.4 |
| 3 | SBE3-14 | 1030 | −48.2 |
| 4 | SBP3-14 | 1673 | −48.8 |
| 5 | SB3-8 bis | 1488 | −48.0 |
| 6 | SB3-MIM | 6556 | −30.5 |
| 7 | SB3-BIM | 2305 | −36.8 |
| 8 | SB3-Cy | 2617 | −56.0 |
| 9 | SB3-Py | 3472 | −60.9 |
| 10 | SB4-14 | 1268 | −44.7 |
| 11 | SB3-4 | 5371 | −98.8 |
| 12 | SB3-4 Tris | 3735 | −48.5 |
Viscosity values observed in SBR3-14 series followed a good linear correlation with Arrhenius law. The most viscous system in this class is the SB3-14–CSA mixture (2161 cP, entry 2), while SBE3-14–CSA and SBP3-14–CSA mixtures showed values of 1030 cP and 1673 cP respectively (entry 3 and 4). The two small methyl groups on ammonium in SB3-14 mixture provoked a strong interaction and therefore very low vacancies radius. The two ethyl groups in SBE3-14–CSA mixture are smaller than bulky n-propyl in SBP3-14 DES, but the propyl can lead to van der Waals interactions. The imidazole-based mixtures (SB3-MIM and SB3-BIM–CSA) showed a non-linear correlation. The plot showed two parted linear trends for both the mixtures, probably due to system rearrangements. The values of viscosity observed for this class were higher than the ones of SBR3-14 series (6556 cP and 2305 cP respectively for SB3-MIM–CSA and SB3-BIM, entry 6 and 7). This is due to the aromatic imidazolium ring that determines a tighter packing of the molecules and smaller vacancies. The butyl chain of SB3-BIM determines a lower symmetry and higher radius of vacancies and therefore lower viscosity than methyl of SB3–MIM mixture. SB3-4–CSA mixture became more viscous than SB3-4 Tris at temperatures higher than 85 °C (5371 cP for SB3-4 and 3735 for SB3-4 Tris, entry 11 and 12) due to higher order in the liquid phase of SB3-4 for its smaller dimensions. SB3-Py–CSA mixture is more viscous (3472 cP, entry 9) than SB3-Cy–CSA mixture (Table 2 entry 8) probably due to the planarity of the system. SB3-MIM structure has also a planar aromatic group, and the viscosities are similar to the one of SB3-Py. The SB3-8 bis mixture showed a low viscosity value (1488 cP, entry 5). This seemed surprising if compared to the one of SB3-12–CSA (3025 cP, entry 1) for their alkyl portions, but the two alkyl chains in SB3-8 bis have more conformational degree of freedom compared to the longer one of SB3-14. The lowest viscosity observed in our set was for SB4-14–CSA mixture (1268 cP, entry 10). In this case the higher dimensions of the spacer may give to the molecules higher mobility, and consequently lower viscosity.
| Entry | DES | σa (μS cm−1) | EΛb (kJ mol−1) |
|---|---|---|---|
| a Values obtained at 75 °C.b Energy of Activation extrapolated at 25 °C. | |||
| 1 | SB3-12 | 23.3 | 33.0 |
| 2 | SB3-14 | 13.8 | 33.3 |
| 3 | SBE3-14 | 34.7 | 34.1 |
| 4 | SBP3-14 | 15.1 | 34.6 |
| 5 | SB3-8 bis | 20.5 | 31.5 |
| 6 | SB3-MIM | 27.8 | 42.1 |
| 7 | SB3-BIM | 39.1 | 38.0 |
| 8 | SB3-Cy | 102.0 | 39.9 |
| 9 | SB3-Py | 30.6 | 44.9 |
| 10 | SB4-14 | 48.3 | 35.3 |
| 11 | SB3-4 | 26.3 | 40.0 |
| 12 | SB3-4 Tris | 8.7 | 42.3 |
The activation energies were calculated at 298 K from eqn (1) via linear correlation of the viscosity values. The four points at lower temperatures were used for correlations of SB–CSA mixtures which showed non-linear trends. The Eη is dependent on the ratio R±/Rh, where R± is the ion radius and Rh is the radius of the vacancies in the liquid. High Eη values mean difficult movement of the molecules in the liquid due to the small dimensions of the vacancies.75,76 As showed in Table 2, an increase of chain length (SB3-12 and SB3-14, entry 1 and 2) led to an increase of Eη values. In SBR3-14 series, the increase of steric hindrance led to an increase of Eη. The presence of two alkyl chains in SB3-8 bis (entry 5) increased the Eη value that became similar to 14-chain sulfobetaines. In the aromatic SB mixtures (entry 6, 7, 9), imidazolium DESs presented similar values of Eη, with a higher energy for SB3-BIM due to the alkyl chain, while the pyridinium SB gave a higher Eη value. SB3-Cy–CSA mixture (entry 8) showed an activation energy higher than the ones with linear chain. The cyclohexyl-, in fact, can provoke good side–side packing in the liquid structure. As well as its high viscosity, SB3-4 DES (entry 11) presented Eη = −98 kJ mol−1, the highest value in our set. The value of Eη for SB3-4 Tris (entry 12) is lower than SB3-4 mixture. The presence of three butyl-chains could provoke a decrease of symmetry. SB4-14–CSA mixture (entry 10) showed an Eη value similar to linear chain SB which was between SB3-14 and SBE3-14 values.
The viscosities of these novel DESs are quite high, maybe due to the strong interaction between the SB and the CSA, and are higher than the viscosities of other trimethylglicine/carboxylic acids DESs that we developed recently.53 However these values are lower than sugar DESs in literature such as ChCl/Xylitol and ChCl/Glucose (5230 cP and 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 cP respectively).20 Viscosity activation energies values are similar to the ones of other DESs in literature.19,20,53
400 cP respectively).20 Viscosity activation energies values are similar to the ones of other DESs in literature.19,20,53
Conductivity
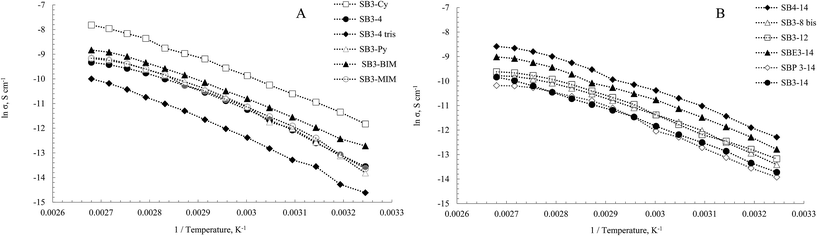
Electrical conductivity of the DESs was measured between 35–100 °C. The profiles of natural logarithm of conductivity vs. 1/T are reported in Fig. 5, the conductivity of the mixtures at 75 °C and the conductivity activation energies at 25 °C are reported in Table 3. All the mixtures showed a good linear correlation with Arrhenius law (eqn (2)).
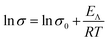 | (2) |
The highest value in our set was SB3-Cy (102 μS cm−1, entry 8) while the lowest was SB3-4 Tris–CSA mixture with a value of 8.7 μS cm−1 (entry 12). In SBR3-14 series (entry 2–4) the mixture SBE3-14–CSA showed the highest conductivity value (34.7, 13.8 and 15.1 μS cm−1 for SBE3-14, SB3-14 and SBP3-14 respectively). In the aromatic series SB3-MIM showed a conductivity lower than SB3-BIM–CSA mixture (27.8 and 39.1 μS cm−1, entry 6 and 7) and SB3-Py showed an intermediate value between them (30.6 μS cm−1, entry 9). The alkyl residues on the side chain didn't impact relevantly on the observed conductivity values. SB3-12–CSA mixture showed, in fact, a value of 23.3 μS cm−1 (entry 1) while SB3-8 bis–CSA showed 20.5 μS cm−1 (entry 5).
The observed conductivity values for these novel DESs are low, even lower than other carboxy-betaine based fluids53 and comparable with AcChCl–urea and ChCl–ZnCl2 mixtures, (17 and 60 μS cm−1 respectively).20 This could be due to the dimensions of the molecules in the DESs, and therefore to their mobility (as also demonstrated by their high viscosity values), but particularly to the strength and to the kind of interaction between the species. The interaction between the HBA and the HBD molecules is quite strong as proved by the Δt of the mixtures, and could be ascribed to a hydrogen bond instead of a complete transfer of the hydrogen from HBD to HBA molecules. This is probably due to the small difference of pKa between the species. This phenomenon is proved also by the values of conductivity: a complete proton transfer causes a formation of an ionic couple, and consequently a protic ionic liquid formation. However the conductivity values observed are too low. The conductivity values of typical Ionic Liquids span, in fact, from 0.0027 to 11 S cm−1, because of the presence of two discrete ionic species.77 Our mixtures showed values between five and seven orders of magnitude lower (moreover at higher temperatures). This indicates the absence of ionic species. The use of liquid mixtures defined as protic ionic liquids is reported in literature, but their conductivity values and their small differences of pKa between the HBA and the HBD molecules suggest that they also could belong to the class of deep eutectic solvents. This is the case of ethanolammonium lactate78 and 1-ethyl imidazolium H2PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79 mixtures that showed conductivity values of 48 μS cm−1 and 11 μS cm−1 respectively.
79 mixtures that showed conductivity values of 48 μS cm−1 and 11 μS cm−1 respectively.
In Table 3 the activation energies of conductivity (EΛ) for these mixtures are reported. This energy is related to the radius of the vacancies in the liquid structure, responsible of mass transport.75,80 An increase of the alkyl chain length (entry 1, 2) led to an increase of EΛ in the same manner of activation energy of viscosity. An increase of the steric hindrance on ammonium atom (entry 2–4) increased activation energy because of the lower mobility of the cation. SB3-8 bis–CSA mixture values are due to the two alkyl chains (entry 5). The cyclohexyl group in SB3-Cy–CSA mixture (entry 8) led to less mobility of the molecules compared to the linear chain for its easier packing. SB3-BIM showed the lowest value in aromatic mixtures (entry 6, 7 and 9): the linear side-chain decreases the ion mobility and decreases also the capability of π–π stacking. The increase of the spacer length in SB4-14–CSA mixture (entry 10) led to an increase of EΛ due to an increase of ionic radius with consequent mobility loss. The ion mobility is responsible also for SB3-4–CSA mixture value that is lower than SB3-4 Tris (entry 11, 12). The values of EΛ observed are similar to the ones of other DESs reported in literature.44,76
Density
The novel DESs developed showed a linear dependence of density with temperature. The density, as well as viscosity and conductivity, relies on the presence of vacancies in the DES network.76 The density variations were detected between 25 °C and 80 °C. The values obtained are reported in Table 4, in Fig. 2S in ESI† section are reported density/temperature profiles.| SB3-12 | SB3-14 | SBE3-14 | SBP3-14 | SB4-14 | SB3-8 bis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T °C | ρ g mL−1 | T °C | ρ g mL−1 | T °C | ρ g mL−1 | T °C | ρ g mL−1 | T °C | ρ g mL−1 | T °C | ρ g mL−1 |
| 25 | 1.18 | 25 | 1.20 | 25 | 1.14 | 25 | 1.12 | 25 | 1.17 | 25 | 1.15 |
| 50 | 1.16 | 50 | 1.18 | 50 | 1.13 | 50 | 1.09 | 45 | 1.15 | 45 | 1.13 |
| 65 | 1.14 | 65 | 1.17 | 65 | 1.11 | 65 | 1.08 | 60 | 1.14 | 60 | 1.12 |
| 80 | 1.13 | 80 | 1.15 | 80 | 1.1 | 80 | 1.06 | 80 | 1.13 | 80 | 1.11 |
| SB3-MIM | SB3-BIM | SB3-4 Tris | SB3-4 | SB3-Cy | SB3-Py | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T °C | ρ g mL−1 | T °C | ρ g mL−1 | T °C | ρ g mL−1 | T °C | ρ g mL−1 | T °C | ρ g mL−1 | T °C | ρ g mL−1 |
| 25 | 1.33 | 25 | 1.23 | 25 | 1.22 | 25 | 1.25 | 25 | 1.27 | 25 | 1.33 |
| 45 | 1.32 | 45 | 1.21 | 45 | 1.21 | 45 | 1.24 | 45 | 1.26 | 45 | 1.31 |
| 60 | 1.31 | 60 | 1.20 | 60 | 1.20 | 60 | 1.23 | 60 | 1.25 | 60 | 1.29 |
| 80 | 1.29 | 80 | 1.17 | 80 | 1.19 | 80 | 1.21 | 80 | 1.21 | 80 | 1.25 |
The highest density variation with temperature was observed in SB3-Py–CSA mixture. The values are similar to the ones observed for SB3-MIM–CSA system. This indicates stronger intermolecular interactions in these mixtures compared with SB3-BIM and SB3-Cy systems. These four systems have values of ρ at 25 °C of 1.33 g mL−1, 1.33 g mL−1, 1.23 g mL−1 and 1.27 g mL−1 respectively. The SBR3-14 series showed density values following the trend methyl > ethyl > n-propyl with ρ values at 25 °C of 1.20 g mL−1, 1.14 g mL−1 and 1.12 g mL−1 for SB3-14, SBE3-14 and SBP3-14 respectively. The increase of steric hindrance suggests the increase of radius of voids and therefore of the lowering in density. SB3-8 bis–CSA mixture showed a low density at 25 °C (1.15 g mL−1): even in this case this value could depend on the radius of the voids, increased by two octyl chains presence in the structure. The same behaviour occurs in SB3-4 and SB3-4 Tris mixtures with 1.25 and 1.22 g mL−1 ρ values. SB4-14–CSA mixture showed values of density similar to SB3-12–CSA mixture (1.17 g mL−1 and 1.18 g mL−1 values respectively at 25 °C) and this could be ascribed to weak intermolecular interactions.
The density values observed are similar for all the DESs we developed, and they are analogous to the ones observed in literature for other DESs such as ChCl–EG systems (1.12 g mL−1)76 or ZnCl2–Acetamide mixtures (1.36 g mL−1).81
Surface tension
The surface tension is a fundamental property of a solvent, it is directly related to the radius of voids and it is a measure of cohesive forces in liquid on the surface.75 The surface tension values observed for our SB–CSA mixtures at 25 °C are reported in Table S1 in ESI† section. All the values observed are lower than water value (71.5 mN m−1). The lowest value observed in our set is for SB3-12–CSA mixture (25.1 mN m−1, entry 1). In SBR3-14 series, SBE3-14–CSA mixture showed the lowest value (36.5, 33.6, 34.8 mN m−1 for SB3-14, SBE3-14 and SBP3-14 respectively, entry 2, 3, 4). This could be due to van der Waals interactions in propyl group and small dimensions of methyl group: the same phenomenon was also observed in viscosity, density and conductivity measures in this work. In the aromatic SB–CSA mixtures (36.5, 32.9, 33.4 mN m−1, entry 6, 7, 10) the values followed the trend SB3-BIM < SB3-Py < SB3-MIM due to the same reasons. SB3-8 bis–CSA mixture (entry 5) showed a value of 37.5 mN m−1, that is higher than the other single-chain SB. The tetramethylene spacer in SB4-14–CSA mixture (entry 8, 33.8 mN m−1) provokes a lowering in surface tension compared to the one of SB3-14–CSA mixture. SB3-4 Tris–CSA mixture (entry 12) showed a value of 42.1 mN m−1, lower than SB3-4 mixture (entry 11, 43.3 mN m−1), confirming the role of the dimensions of the molecules on surface tension values.The surface tension values observed for these novel DESs are similar to ChCl–alcohol mixtures reported in literature.45,82
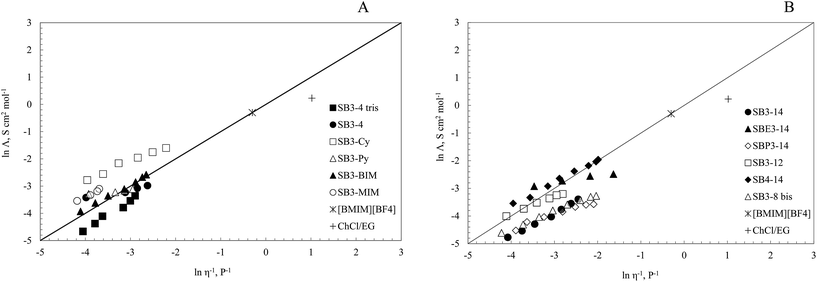
Ionicity
The ionicity of DESs is an evaluation of the free charges fraction at a specific temperature. This property is directly correlated to the low vapour pressure of these liquids that is lower, in fact, for higher charge fraction systems. Different techniques were used in literature to evaluate ionicity, such as thermal properties changes,83,84 NMR,85,86 and IR spectroscopy.83 The Walden plot represents a simple and qualitative method that can be used in this field to evaluate the ionicity of a DES.87,88 It is based on the assumption that strong electrolytes such as KCl follow Walden's law: the molar conductivity (Λ) is linearly dependent on the viscosity (η) (eqn (3)).| Λη = Const | (3) |
Angell and co-workers proposed to use the Walden plot as a qualitative estimation of the ionicity of the media.87 According to this, we made Walden plots of ln Λ/ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η−1 of our twelve DESs. In this chart the diagonal represents the theoretical behavior of KCl in water, considering it fully dissociated and with equal mobility ions. The mixtures that have ln
η−1 of our twelve DESs. In this chart the diagonal represents the theoretical behavior of KCl in water, considering it fully dissociated and with equal mobility ions. The mixtures that have ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Λ/ln
Λ/ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η−1 values close to that diagonal, can be considered “good ionic liquids”, the ones far from these values in the lower portion can be considered “poor ionic liquids”. Non-ionic liquids are in the lower portion, very distant from the diagonal. Super-ionic liquids can be found on the upper side of the plot.79,89,90
η−1 values close to that diagonal, can be considered “good ionic liquids”, the ones far from these values in the lower portion can be considered “poor ionic liquids”. Non-ionic liquids are in the lower portion, very distant from the diagonal. Super-ionic liquids can be found on the upper side of the plot.79,89,90
In Fig. 6 are reported the Walden plots of the investigated DESs. For comparison with our systems, in the same figure are reported in both panels [BMIM][BF4] and ChCl–EG DES as model respectively for ionic liquid systems and a choline-based mixtures.82,87 All our twelve mixtures showed values close to reference line, so they can be considered “good ionic liquids”.
Ionic liquids are close to the reference line in the Walden plot.91 Protic ionic liquids (PILs), formed by proton transfer, are commonly far from the reference line in the plot,79 and therefore they can be considered as “poor ionic liquids”. Some protic ionic liquids, however, are closer to the reference line when the ΔpKa of the molecules forming them is high (about 10).91 On the contrary, in these novel DESs the pKa values of the two species are similar but the positions in the Walden plot are close to the reference line. In our systems the overall charges are the same as the ones of an IL, but the molecules presented a lower mobility due to an interaction between them that could be ascribed to a hydrogen bond. The proton could be located, in fact, between the HBA and the HBD molecules, and therefore leading to partial charges formation. The low conductivity values showed by these mixtures also confirmed this phenomenon. The position of these novel mixtures in the plot, down on the left, is also an evidence of their properties: their behavior could be ascribed to highly diluted solutions of electrolytes and the charge transfer between the species is very low. Both PILs (with a complete charge transfer) and ILs (with discrete ionic species) are on the right portion on the Walden plot: this indicates our mixtures to be DESs instead of these sorts of mixtures. To our knowledge it is the first time that novel media are shown in this left portion of a Walden plot.
FTIR bioassay
Fourier transform infrared spectroscopy (FTIR) has been applied in the last two decades in microbiological studies,73 initially to define the relations among strains and species,92 then to dereplicate and to define possible correlations between FTIR and other molecular descriptors.93 The low cost in consumables, the reproducibility and the rapidity were considered key factors in this application.94From a previous analysis, it has emerged that these compounds brought 100% of cell mortality after little exposure times (data not shown). Preliminary microbiological tests showed a very rapid decrease of cell viability after few minutes of exposition to the tested mixtures. We observed the same behavior in our previous study regarding trimethylglicine and carboxylic acids DESs53 so we developed FTIR measures in the same manner. It was hypothesized that the high concentration of the DESs caused a very rapid exit of the yeast cell water and thus their immediate inactivation. In order to test this hypothesis, CaCl2 was chosen as a dehydrating agent known not to be toxic, in order to avoid the simultaneous presence of cyto-toxic and dehydration effect.
The effects exerted by all these mixtures were evaluated by means of average Response Spectra (ARS), calculated for amphiphilic SBs–CSA and aromatic–aliphatic SBs–CSA mixtures (Fig. 7).
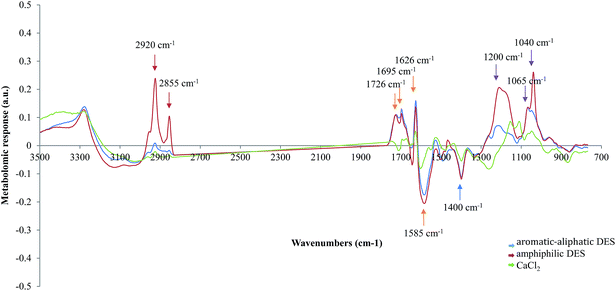
ARSs of aromatic–aliphatic SBs DESs showed intensity very similar to that of CaCl2 with a similar shape in the fatty acids (W1, 3200–2800 cm−1) and mixed region (W3, 1500–1200 cm−1). In the amide I region (W2, 1800–1500 cm−1) aromatic–aliphatic and amphiphilic SBs DESs showed almost identical ARSs, whereas in the carbohydrates and typing region (W5, 900–700 cm−1) the ARSs of the tested compounds diverged. Amphiphilic SBs DESs showed always no lower ARSs intensity than aromatic and aliphatic ones. The ARSs shapes of these two compound classes were similar throughout the whole spectrum except in W4 (1200–900 cm−1) and W5.
Saccharomyces cerevisiae cells showed a metabolomic response in W1 region mainly when challenged by amphiphilic compounds, with two peaks around 2920 and 2855 cm−1 corresponding to asymmetric and symmetric stretching of CH2, respectively.95 The intensity of these bands was positively related to the amount of lipids in bacterial cells.96 In the amides region (1800–1500 cm−1) the two ARSs displayed a similar trend with an almost identical metabolomic response. Relevant peaks were observed at 1726 cm−1, 1695 cm−1 and 1626 cm−1. Many authors attribute these bands to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of lipid esters, antiparallel plated sheet and β-turns in Amide I and protein β-sheet, respectively.95,97,98 It has been reported that an increase in the number of β-sheets in the cell proteins and in the number of strands in the β-sheets, is linked to protein denaturation,98 as expected by proteins undergoing a fast dehydration.99 This was also observed with a negative peak, common to all DESs, detected at 1585 cm−1, corresponding to the amino acids side chain vibration.100 Another negative band was observed in the mixed region (W3) around 1400 cm−1 (C
O of lipid esters, antiparallel plated sheet and β-turns in Amide I and protein β-sheet, respectively.95,97,98 It has been reported that an increase in the number of β-sheets in the cell proteins and in the number of strands in the β-sheets, is linked to protein denaturation,98 as expected by proteins undergoing a fast dehydration.99 This was also observed with a negative peak, common to all DESs, detected at 1585 cm−1, corresponding to the amino acids side chain vibration.100 Another negative band was observed in the mixed region (W3) around 1400 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ν sym of COO−).101
O ν sym of COO−).101
Amphiphilic SBs mixtures determined a more intense response than aromatic–aliphatic ones in carbohydrates region (W4), around 1200 cm−1 (C–O–C vibrations), 1065 cm−1 (DNA and RNA C–C ν skeletal cis conformation)95 and 1040 cm−1 (C–O stretch of nucleic acids).102
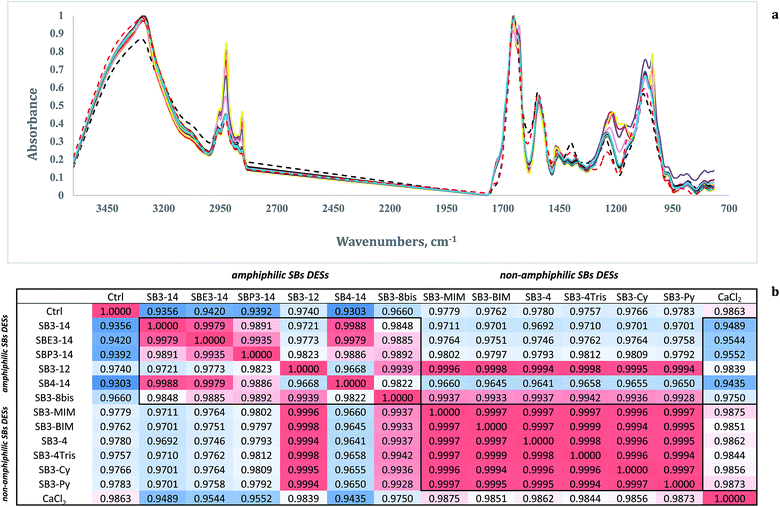
In Fig. 8 (panel a) are reported the average normalized spectra from cells treated with DESs and CaCl2. In panel b of the same figure are reported Pearson's correlations calculated among average spectra.
The Pearson's correlations of the spectra (panel b) showed again that the effects of the DESs on the yeast cells could be grouped in two main classes, corresponding to amphiphilic SBs–CSA mixtures (SB3-14, SBE3-14, SBP3-14, SB3-12, SB4-14, SB3-8 bis) and aromatic–aliphatic SBs–CSA mixtures (SB3-MIM, SB3-MIM, SB3-4, SB3-4 Tris, SB3-Cy, SB3-Py). For this reason the two classes are boxed in the table.
The first group showed correlations ranging between 0.9721 and 0.9988 and from 0.9435 to 0.9750 with CaCl2. The aromatic–aliphatic SBs showed high levels of correlation among themselves (ranging from 0.9994 to 0.9999) and with CaCl2 (from 0.9844 to 0.9875). These figures indicated that the mode of action of the aromatic–aliphatic SBs is likely due to the rapid dehydration of the yeast cells, whereas the amphiphilic SBs could challenge the cells with different mechanisms, including the dehydration itself.
Amphiphilic sulfobetaines–CSA mixtures showed a different behavior. Structure-effect considerations can be made in this class of mixtures. The chain length, and therefore the amphiphilic behaviour of the molecules, had an impact on the effect of the mixtures on the yeast cells. The 14-methylene chain compounds showed a high correlation among them and different from the aromatic–aliphatic mixtures, while 12-methylene chains and 8 bis-methylene chains showed a behaviour that was more similar to the non-amphiphilic sulfobetaines mixtures. In recent studies we observed the same effect of the chain length in FTIR in N-alkyl-tropinium surfactants.63,103 14-methylene, or longer,104 chains showed high biocidal effects on Saccharomyces cerevisiae and Candida albicans yeasts and Escherichia coli and Listeria innocua bacteria, while 12-methylene-chain surfactants showed weaker effects.
Taken together, these data suggest that these novel DESs exert a dehydration effect as CaCl2, as shown by the similar shape of the first three spectral regions. Remarkably, the effect of DESs was always superior to that of calcium chloride salt, indicating more affinity of these compounds to water. The fact that the ARSs shapes in the fatty acid region were almost identical for CaCl2 and DESs can be justified by the fact that this region refers to highly hydrophobic compounds, less likely to react to different amounts of water in the cells. Vice versa the overall shape similarity of ARSs in W2 and W3 regions indicates that the dehydration caused strong variations in the protein asset, which is indeed very sensitive to free water levels. The differences found in W4 and W5 between CaCl2 and DESs could be ascribed to some post mortem effects caused specifically by DESs. One possible explanation is that the violent dehydration triggered by DESs broke part of the internal cell compartmentalization with subsequent fast release of small molecules such as carbohydrates of W4 and, probably, the free amino acids sometimes detected in W5.105,106
Sulfobetaine DES as reaction medium for chalcone synthesis
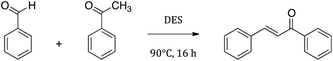
One of the most important applications of novel liquid mixtures is represented by their use as reaction media. In this field DESs have been largely used in the recent years as effective solvents for their properties.27–31 In order to determine the acid properties of the novel DESs presented in this work, we have tested one of our novel mixtures as solvent and Brønsted catalyst in chalcone synthesis from benzaldehyde and acetophenone as probe reaction (Fig. 9).These novel DESs have also a catalytic behaviour in this Claisen–Schmidt condensation due to the acid properties of CSA composing it. The reaction, in fact, is usually carried out in basic or acid catalysis conditions, with the addition of e.g. sodium or potassium hydroxide, alkali alcoholates,107,108 solid basic catalysers109,110 or concentrated sulfuric acid.111 SB3-Cy–CSA deep eutectic solvent was tested for this reaction.
Temperature and reaction times were varied to optimize conversion and yield of the reaction. A temperature of 90 °C and a reaction time of 16 h were chosen as optimal after a series of experiments (data not shown). In these conditions we obtained a weighed yield of purified chalcone of 70% with a conversion of 96%. Subsequent experiments were carried out to determine the DES recycling capability. The DES is, in fact, highly soluble in water, while the chalcone is poorly soluble in it, so the DES could be easily removed by washing the mixture with H2O then drying it under vacuum after each cycle. The yields remained constant for over 4 consecutives reaction cycles.
The same reaction conducted in acid ionic liquids as reported in literature led to almost the same yields but with higher temperatures (140 °C) and into inert atmosphere.112,113 The use of other reaction media led to higher yields but the use of harmful acids or bases as catalysts was needed and therefore their disposal.110,114 These are not necessary in the case of the use of these DESs. These data promote the “greenness” of these mixtures and suggest further investigations on their properties as promising reaction media. Further studies on a wide set of acetophenones and benzaldehydes molecules are currently undergoing in our laboratories, in order to determine the versatility of these novel media.
Conclusions
Twelve novel deep eutectic solvents were prepared and characterized. They are formed by mixtures of (1S)-(+)-10-camphorsulfonic acid and aliphatic, aromatic and amphiphilic sulfobetaines. Their melting points span from −5 to 19 °C, this indicates that the driving forces leading to liquid systems are almost identical for all the mixtures. They are liquids at room temperature, so we can name these mixtures RTDESs (room temperature deep eutectic solvents). The DESs were characterized in terms of their viscosity, conductivity (and therefore their ionicity via Walden plot), density, surface tension and toxicity on eukaryotic model cells. The collected data suggest that the interaction between CSA and the sulfobetaine can be ascribed as a hydrogen bond. The low conductivity values, the liquid formation even at 0.2 molar fraction of the acid and the small differences of pKa values between the two species, in fact, suggest that the proton is not completely transferred from HBD to HBA molecules. For these reasons these mixtures cannot be considered ionic liquids. To our knowledge, their position on the Walden plot, in the left portion close to the diagonal, has not yet been observed for other DESs or ionic liquid systems. It indicates that these mixtures have ionicity similar to highly diluted electrolyte solutions. The values of their viscosity, their surface tension and their density are similar to other DESs systems in literature.The FTIR-based bioassay that we developed to determine the toxicity of these mixtures on eukaryotic model cells (Saccharomyces cerevisiae) showed that these novel DESs exert a dehydration effect, highly correlated to a well-known non-toxic agent such as CaCl2. This supports these DESs as promising green media. The amphiphilic SBs mixtures showed a stronger effect on the cells; a structure–activity trend can be described for this class of mixtures. 14-Methylene chain SBs DESs showed a stronger activity, the smaller methylene chain SBs DESs (12-methylene chain and 8-bis-methylene chain) showed a similar effect but more correlated with aliphatic and aromatic SBs DESs. This is probably due to their lower hydrophobicity.
The use of these novel DESs as reaction media with catalytic properties was accomplished by the use of SB-Cy–CSA DES in chalcone synthesis, a well-known and studied acid-catalysed reaction. This DES showed acid catalytic properties, yields of 70% and conversion of 96%, recycling capabilities in subsequent reactions and prevented the use of harmful acid catalysts. These preliminary data promote the “greenness” of these mixtures and suggest further investigations in this field.
Acknowledgements
The authors thank the Ministero per l'Università e la Ricerca Scientifica e Tecnologica, MIUR (Rome, Italy) [PRIN “Programmi di Ricerca di Interesse Nazionale” 2010–2011, n. 2010FM738P] and Regione Umbria (POR FSE 2007–2013, Risorse CIPE, Perugia, Italy) for fundings.References
- R. A. Sheldon, Green Chem., 2005, 7, 267–278 RSC.
- S. R. Cicco, G. M. Farinola, C. Martinelli, F. Naso and M. Tiecco, Eur. J. Org. Chem., 2010, 2275–2279 CrossRef CAS.
- C. Reichardt and T. Welton, Solvents and solvent effects in organic chemistry, John Wiley & Sons, 2011 Search PubMed.
- P. Wasserscheid and T. Welton, Ionic liquids in synthesis, Wiley Online Library, 2008 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- L. P. Rebelo, J. N. Canongia Lopes, J. M. Esperança and E. Filipe, J. Phys. Chem. B, 2005, 109, 6040–6043 CrossRef CAS PubMed.
- M. Matzke, S. Stolte, K. Thiele, T. Juffernholz, J. Arning, J. Ranke, U. Welz-Biermann and B. Jastorff, Green Chem., 2007, 9, 1198–1207 RSC.
- J. Ranke, K. Mölter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffmann, B. Ondruschka, J. Filser and B. Jastorff, Ecotoxicol. Environ. Saf., 2004, 58, 396–404 CrossRef CAS.
- P. Stepnowski, A. Skladanowski, A. Ludwiczak and E. Laczyńska, Hum. Exp. Toxicol., 2004, 23, 513–517 CAS.
- R. J. Bernot, E. E. Kennedy and G. A. Lamberti, Environ. Toxicol. Chem., 2005, 24, 1759–1765 CrossRef CAS.
- Y. Fukaya, Y. Iizuka, K. Sekikawa and H. Ohno, Green Chem., 2007, 9, 1155–1157 RSC.
- Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550–9570 RSC.
- A. Wolfson, A. Snezhko, T. Meyouhas and D. Tavor, Green Chem. Lett. Rev., 2012, 5, 7–12 CrossRef CAS.
- J. Yang, J.-N. Tan and Y. Gu, Green Chem., 2012, 14, 3304–3317 RSC.
- I. T. Horváth, Green Chem., 2008, 10, 1024–1028 RSC.
- M. Virot, V. Tomao, C. Ginies, F. Visinoni and F. Chemat, J. Chromatogr. A, 2008, 1196, 147–152 CrossRef PubMed.
- C. Denis, B. Laignel, D. Plusquellec, J.-Y. Le Marouille and A. Botrel, Tetrahedron Lett., 1996, 37, 53–56 CrossRef CAS.
- B. Zhou, J. Yang, M. Li and Y. Gu, Green Chem., 2011, 13, 2204–2211 RSC.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- M. A. Kareem, F. S. Mjalli, M. A. Hashim and I. M. AlNashef, J. Chem. Eng. Data, 2010, 55, 4632–4637 CrossRef CAS.
- A. P. Abbott, K. El Ttaib, G. Frisch, K. J. McKenzie and K. S. Ryder, Phys. Chem. Chem. Phys., 2009, 11, 4269–4277 RSC.
- M. Figueiredo, C. Gomes, R. Costa, A. Martins, C. M. Pereira and F. Silva, Electrochim. Acta, 2009, 54, 2630–2634 CrossRef CAS PubMed.
- E. Gómez, P. Cojocaru, L. Magagnin and E. Valles, J. Electroanal. Chem., 2011, 658, 18–24 CrossRef PubMed.
- C. A. Nkuku and R. J. LeSuer, J. Phys. Chem. B, 2007, 111, 13271–13277 CrossRef CAS PubMed.
- H. G. Morrison, C. C. Sun and S. Neervannan, Int. J. Pharm., 2009, 378, 136–139 CrossRef CAS PubMed.
- Z. Chen, W. Zhu, Z. Zheng and X. Zou, J. Fluorine Chem., 2010, 131, 340–344 CrossRef CAS PubMed.
- S. Handy and K. Lavender, Tetrahedron Lett., 2013, 54, 4377–4379 CrossRef CAS PubMed.
- U. B. Patil, A. S. Singh and J. M. Nagarkar, RSC Adv., 2014, 4, 1102–1106 RSC.
- B. S. Singh, H. R. Lobo and G. S. Shankarling, Catal. Commun., 2012, 24, 70–74 CrossRef CAS PubMed.
- C. Vidal, F. J. Suárez and J. García-Álvarez, Catal. Commun., 2014, 44, 76–79 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, K. J. McKenzie and S. U. Obi, J. Chem. Eng. Data, 2006, 51, 1280–1282 CrossRef CAS.
- Y. Dai, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chem., 2013, 85, 6272–6278 CrossRef CAS PubMed.
- F. S. Oliveira, A. B. Pereiro, L. P. Rebelo and I. M. Marrucho, Green Chem., 2013, 15, 1326–1330 RSC.
- J. T. Gorke, F. Srienc and R. J. Kazlauskas, Chem. Commun., 2008, 1235–1237 RSC.
- D. Lindberg, M. de la Fuente Revenga and M. Widersten, J. Biotechnol., 2010, 147, 169–171 CrossRef CAS PubMed.
- H. Zhao, G. A. Baker and S. Holmes, J. Mol. Catal. B: Enzym., 2011, 72, 163–167 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- K. Radošević, M. C. Bubalo, V. G. Srček, D. Grgas, T. L. Dragičević and I. R. Redovniković, Ecotoxicol. Environ. Saf., 2015, 112, 46–53 CrossRef PubMed.
- S. B. Phadtare and G. S. Shankarling, Green Chem., 2010, 12, 458–462 RSC.
- A. Zhu, T. Jiang, B. Han, J. Zhang, Y. Xie and X. Ma, Green Chem., 2007, 9, 169–172 RSC.
- A. P. Abbott, G. Capper, K. J. McKenzie and K. S. Ryder, J. Electroanal. Chem., 2007, 599, 288–294 CrossRef CAS PubMed.
- W. Guo, Y. Hou, S. Ren, S. Tian and W. Wu, J. Chem. Eng. Data, 2013, 58, 866–872 CrossRef CAS.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- A. P. Abbott, R. C. Harris, K. S. Ryder, C. D'Agostino, L. F. Gladden and M. D. Mantle, Green Chem., 2011, 13, 82–90 RSC.
- A. Hayyan, F. S. Mjalli, I. M. AlNashef, T. Al-Wahaibi, Y. M. Al-Wahaibi and M. A. Hashim, Thermochim. Acta, 2012, 541, 70–75 CrossRef CAS PubMed.
- Z. Maugeri and P. D. de María, RSC Adv., 2012, 2, 421–425 RSC.
- D. Carriazo, M. a. C. Gutiérrez, M. L. Ferrer and F. del Monte, Chem. Mater., 2010, 22, 6146–6152 CrossRef CAS.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- F. De Zwart, S. Slow, R. Payne, M. Lever, P. George, J. Gerrard and S. Chambers, Food Chem., 2003, 83, 197–204 CrossRef CAS.
- G. Qu, J. Cheng, J. Wei, T. Yu, W. Ding and H. Luan, J. Surfactants Deterg., 2011, 14, 31–35 CrossRef CAS PubMed.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Green Chem., 2012, 14, 2153–2157 RSC.
- F. Cardellini, M. Tiecco, R. Germani, G. Cardinali, L. Corte, L. Roscini and N. Spreti, RSC Adv., 2014, 4, 55990–56002 RSC.
- F. Cardellini, L. Brinchi, R. Germani and M. Tiecco, Synth. Commun., 2014, 44, 3248–3256 CrossRef CAS.
- V. De Santi, F. Cardellini, L. Brinchi and R. Germani, Tetrahedron Lett., 2012, 53, 5151–5155 CrossRef CAS PubMed.
- L. Brinchi, R. Germani, E. Braccalenti, N. Spreti, M. Tiecco and G. Savelli, J. Colloid Interface Sci., 2010, 348, 137–145 CrossRef CAS PubMed.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS PubMed.
- Y. Gu, F. Shi and Y. Deng, Catal. Commun., 2003, 4, 597–601 CrossRef CAS PubMed.
- J. Gui, X. Cong, D. Liu, X. Zhang, Z. Hu and Z. Sun, Catal. Commun., 2004, 5, 473–477 CrossRef CAS PubMed.
- H. Xing, T. Wang, Z. Zhou and Y. Dai, Ind. Eng. Chem. Res., 2005, 44, 4147–4150 CrossRef CAS.
- L. Corte, P. Rellini, L. Roscini, F. Fatichenti and G. Cardinali, Anal. Chim. Acta, 2010, 659, 258–265 CrossRef CAS PubMed.
- M. Tiecco, G. Cardinali, L. Roscini, R. Germani and L. Corte, Colloids Surf., B, 2013, 111, 407–417 CrossRef CAS PubMed.
- L. Corte, M. Tiecco, L. Roscini, R. Germani and G. Cardinali, Colloids Surf., B, 2014, 116, 761–771 CrossRef CAS PubMed.
- N. Spreti, A. Bartoletti, P. D. Profio, R. Germani and G. Savelli, Biotechnol. Prog., 1995, 11, 107–111 CrossRef CAS PubMed.
- Y. Tian, X. Meng and L. Shi, Ind. Eng. Chem. Res., 2013, 52, 6655–6661 CrossRef CAS.
- K. Funabiki, T. Komeda, Y. Kubota and M. Matsui, Tetrahedron, 2009, 65, 7457–7463 CrossRef CAS PubMed.
- N. Expert-Bezançon, T. Rabilloud, L. Vuillard and M. E. Goldberg, Biophys. Chem., 2003, 100, 469–479 CrossRef.
- M. Galin, A. Chapoton and J.-C. Galin, J. Chem. Soc., Perkin Trans. 2, 1993, 545–553 RSC.
- M.-L. Wang and W.-H. Chen, Chem. Eng. Commun., 2007, 194, 19–36 CrossRef CAS.
- C. P. Kurtzman and C. J. Robnett, Antonie van Leeuwenhoek, 1998, 73, 331–371 CrossRef CAS.
- M. Essendoubi, D. Toubas, M. Bouzaggou, J.-M. Pinon, M. Manfait and G. D. Sockalingum, Biochim. Biophys. Acta, Gen. Subj., 2005, 1724, 239–247 CrossRef CAS PubMed.
- W. E. Huang, D. Hopper, R. Goodacre, M. Beckmann, A. Singer and J. Draper, J. Microbiol. Methods, 2006, 67, 273–280 CrossRef CAS PubMed.
- D. Naumann, D. Helm and H. Labischinski, Nature, 1991, 351, 81–82 CrossRef CAS PubMed.
- A. P. Abbott, ChemPhysChem, 2004, 5, 1242–1246 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper and S. Gray, ChemPhysChem, 2006, 7, 803–806 CrossRef CAS PubMed.
- A. P. Abbott, J. C. Barron, K. S. Ryder and D. Wilson, Chem.–Eur. J., 2007, 13, 6495–6501 CrossRef CAS PubMed.
- S. Zhang, N. Sun, X. He, X. Lu and X. Zhang, J. Phys. Chem. Ref. Data, 2006, 35, 1475–1517 CrossRef CAS PubMed.
- T. L. Greaves, A. Weerawardena, C. Fong, I. Krodkiewska and C. J. Drummond, J. Phys. Chem. B, 2006, 110, 22479–22487 CrossRef CAS PubMed.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed.
- A. P. Abbott, ChemPhysChem, 2005, 6, 2502–2505 CrossRef CAS PubMed.
- K. Shahbaz, S. Baroutian, F. Mjalli, M. Hashim and I. AlNashef, Thermochim. Acta, 2012, 527, 59–66 CrossRef CAS PubMed.
- A. P. Abbott, R. C. Harris and K. S. Ryder, J. Phys. Chem. B, 2007, 111, 4910–4913 CrossRef CAS PubMed.
- B. Nuthakki, T. L. Greaves, I. Krodkiewska, A. Weerawardena, M. I. Burgar, R. J. Mulder and C. J. Drummond, Aust. J. Chem., 2007, 60, 21–28 CrossRef CAS.
- H. Matsuoka, H. Nakamoto, M. A. B. H. Susan and M. Watanabe, Electrochim. Acta, 2005, 50, 4015–4021 CrossRef CAS PubMed.
- D. R. MacFarlane, J. M. Pringle, K. M. Johansson, S. A. Forsyth and M. Forsyth, Chem. Commun., 2006, 1905–1917 RSC.
- A. Noda, M. A. B. H. Susan, K. Kudo, S. Mitsushima, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2003, 107, 4024–4033 CrossRef CAS.
- W. Xu, E. I. Cooper and C. A. Angell, J. Phys. Chem. B, 2003, 107, 6170–6178 CrossRef CAS.
- C. Schreiner, S. Zugmann, R. Hartl and H. J. Gores, J. Chem. Eng. Data, 2009, 55, 1784–1788 CrossRef.
- C. A. Angell, N. Byrne and J.-P. Belieres, Acc. Chem. Res., 2007, 40, 1228–1236 CrossRef CAS PubMed.
- K. Ueno, H. Tokuda and M. Watanabe, Phys. Chem. Chem. Phys., 2010, 12, 1649–1658 RSC.
- M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411–15419 CrossRef CAS PubMed.
- C. A. Rebuffo, J. Schmitt, M. Wenning, F. von Stetten and S. Scherer, Appl. Environ. Microbiol., 2006, 72, 994–1000 CrossRef CAS PubMed.
- H. Zhao, Y. Kassama, M. Young, D. B. Kell and R. Goodacre, Appl. Environ. Microbiol., 2004, 70, 6619–6627 CrossRef CAS PubMed.
- L. Roscini, L. Corte, L. Antonielli, P. Rellini, F. Fatichenti and G. Cardinali, Analyst, 2010, 135, 2099–2105 RSC.
- C. Yu and J. Irudayaraj, Biopolymers, 2005, 77, 368–377 CrossRef CAS PubMed.
- S. M. Ede, L. M. Hafner and P. M. Fredericks, Appl. Spectrosc., 2004, 58, 317–322 CrossRef CAS PubMed.
- D. Toubas, M. Essendoubi, I. Adt, J.-M. Pinon, M. Manfait and G. D. Sockalingum, Anal. Bioanal. Chem., 2007, 387, 1729–1737 CrossRef CAS PubMed.
- A. Barth, Biochim. Biophys. Acta, Gen. Subj., 2007, 1767, 1073–1101 CrossRef CAS PubMed.
- C. Pénicaud, S. Landaud, F. Jamme, P. Talbot, M. Bouix, S. Ghorbal and F. Fonseca, PLoS One, 2014, 9, e111138 Search PubMed.
- D. Naumann, Encyclopedia of analytical chemistry, 2000 Search PubMed.
- K. Maquelin, C. Kirschner, L.-P. Choo-Smith, N. van den Braak, H. P. Endtz, D. Naumann and G. Puppels, J. Microbiol. Methods, 2002, 51, 255–271 CrossRef CAS.
- M. J. German, A. Hammiche, N. Ragavan, M. J. Tobin, L. J. Cooper, S. S. Matanhelia, A. C. Hindley, C. M. Nicholson, N. J. Fullwood and H. M. Pollock, Biophys. J., 2006, 90, 3783–3795 CrossRef CAS PubMed.
- L. Corte, M. Tiecco, L. Roscini, S. De Vincenzi, C. Colabella, R. Germani, C. Tascini and G. Cardinali, PLoS One, 2014, 10, e0115275 Search PubMed.
- M. Tiecco, L. Corte, L. Roscini, C. Colabella, R. Germani and G. Cardinali, Chem.-Biol. Interact., 2014, 218, 20–27 CrossRef CAS PubMed.
- M. Wolpert and P. Hellwig, Spectrochim. Acta, Part A, 2006, 64, 987–1001 CrossRef PubMed.
- A. Barth, Prog. Biophys. Mol. Biol., 2000, 74, 141–173 CrossRef CAS.
- G. W. Cave and C. L. Raston, Chem. Commun., 2000, 2199–2200 RSC.
- A. Mitsutani, Catal. Today, 2002, 73, 57–63 CrossRef CAS.
- M. Climent, A. Corma, S. Iborra and J. Primo, J. Catal., 1995, 151, 60–66 CrossRef CAS.
- M. Climent, A. Corma, S. Iborra and A. Velty, J. Catal., 2004, 221, 474–482 CrossRef CAS PubMed.
- L. M. Baigrie, R. A. Cox, H. Slebocka-Tilk, M. Tencer and T. T. Tidwell, J. Am. Chem. Soc., 1985, 107, 3640–3645 CrossRef CAS.
- J. Shen, H. Wang, H. Liu, Y. Sun and Z. Liu, J. Mol. Catal. A: Chem., 2008, 280, 24–28 CrossRef CAS PubMed.
- F. Dong, C. Jian, F. Zhenghao, G. Kai and L. Zuliang, Catal. Commun., 2008, 9, 1924–1927 CrossRef PubMed.
- D. G. Powers, D. S. Casebier, D. Fokas, W. J. Ryan, J. R. Troth and D. L. Coffen, Tetrahedron, 1998, 54, 4085–4096 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03932k |
| This journal is © The Royal Society of Chemistry 2015 |