Enhancement of the outdoor stability of dye-sensitized solar cells by a spectrum conversion layer with 1,8-naphthalimide derivatives†
Abstract
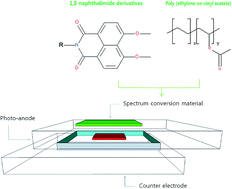
As DSSCs are vulnerable to continuous irradiation of Ultra Violet (UV) light, for outdoor stability, a UV cut-off filter is vital to shield the UV light under outdoor conditions. Unfortunately, the large drop in photo-conversion efficiency by the UV cut-off is inevitable to maintain the outdoor stability. Herein, we propose a novel UV conversion layer using a unique combination of spectrum conversion materials with UV absorbing 1,8-naphthalimide derivatives in poly(ethylene-co-vinyl acetate) on the photo-anode. This functional layer has shown unique characteristics which form exciton complex molecules at high concentration under UV absorption. As a result of converting absorbed UV light to a visible light source for the sensitizing dye, the relative efficiency of the proposed DSSCs have shown comparable initial photo-conversion efficiency to bare DSSC and maintained 18% higher relative photo-conversion efficiency after 48 days outdoor conditions compared to a DSSC using a commercial UV cut-off filter.

 Please wait while we load your content...
Please wait while we load your content...