A general method for the preparation of a thickness-controllable fluoro-containing organic film as a solid lubricant†
Abstract
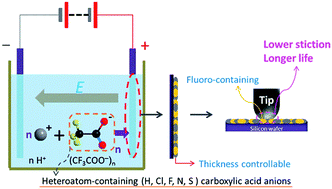
This research presents a general electrophoretic deposition (EPD) method, which offers selective preparation of various heteroatom-containing (i.e., H, Cl, F, N, and S) carboxylic acid molecular (CAM) films directly on silicon wafers, as long as the precursors are water-soluble carboxylic acids. Among them, the as-prepared fluoro-carboxylic acid (trifluoroacetic acid) molecular film with controllable thickness exhibits a longer wear life than self-assembled monolayers (SAMs), along with very low friction coefficient and a superior tolerance to humidity. It may be a promising candidate to replace SAMs as a more wear-resistant solid lubricant in micro/nano electromechanical systems (MEMS/NEMS).

 Please wait while we load your content...
Please wait while we load your content...