Green synthesis, characterization and anticancer activity of luminescent gold nanoparticles capped with apo-α-lactalbumin
Deepthi S. Yarramala,
Sejal Doshi and
Chebrolu P. Rao*
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai – 400 076, India. E-mail: cprao@iitb.ac.in
First published on 30th March 2015
Abstract
A green synthesis was developed in order to prepare protein coated gold nanoparticles using a metal free, α-helical protein, i.e., apo-α-LA, that acts as both the reducing as well as stabilizing agent to result in Au0 nanoparticles from Au3+ which are luminescent and hence can be used for biological imaging, including of cells. The nanoparticles, apo-α-LA-AuNPs, thus synthesized were characterized by multiple techniques, such as, analytical, spectral, microscopy and light scattering, in order to support the presence of NPs in terms of their size and shape, involvement of Au0 and the protein encapsulation and its structural changes upon coating. The 10–16 nm sized apo-α-LA-AuNPs were shown to be non-toxic to healthy cells as studied using normal mouse fibroblast cells (L929). Having found that these NPs are biocompatible and possess structurally altered apo-α-LA protein, the luminescent apo-α-LA-AuNPs were demonstrated to have cytotoxicity towards cancer cells as studied by cell viability tests as well as fluorescence microscopy. While these NPs kill ∼75% of MCF-7 cells, at the same concentration these are capable of killing only ∼30% of HeLa cells, thus exhibiting cell dependency. The present study clearly demonstrates the advantage of the luminescent apo-α-LA-AuNPs in their attack of cancer cells in general and selective killing of breast cancer cells in particular. Thus coating AuNPs with the protein apo-α-LA enhanced their anticancer activity several fold.
Introduction
Recently, noble metal nanoparticles have been studied extensively for a wide range of applications due to their prominent physical, optical and chemical properties.1–4 Gold nanoparticles (AuNPs) have been extensively used for biological and biomedical applications due to their attractive properties like good biocompatibility, photostability and cellular uptake.5–9 AuNPs of 3–50 nm in size are of great interest owing to their image contrast, enhanced Surface Plasmon Resonance (SPR), biocompatibility and ease of synthesis.1,10,11 The Localised Surface Plasmon Resonance (LSPR) band positions of AuNPs are highly sensitive to their size, shape and the capping agent used.12,13 The size and shape controlled synthesis of AuNPs results in emergent or new functions such as size dependent molecular fluorescence, enhanced stability etc., which are useful in various applications like bio-labelling, biomedicine, biosensors and cancer research.14,15 Many researchers reported various methods for size and shape controlled nucleation and growth of AuNPs using external reducing agents and even high temperature or pressure or both.2,16–18 In recent years, the literature has started witnessing reports on the development of green synthesis routes for AuNPs, where a protein acts as the reducing as well as the stabilizing agent.19–22 In the literature, the development of green synthetic routes for luminescent AuNPs is still in its early phase and further applications are yet to be explored.23,24 The utility of AuNPs will be greater if they can be prepared through a green synthetic route under ambient conditions without the use of any external reducing agents. Bovine α-lactalbumin (α-LA) made lethal to cancer cells (BAMLET) is a partially unfolded state of α-LA which is stabilized by oleic acid and is known to cause the cancer cell death through apoptosis while sparing the healthy cells.25,26 The oleic acid molecules in the BAMLET plays an important role to stabilize the partially unfolded molten globular states of α-LA so as it can act against the cancer cells.27,28 Adsorption or binding of α-LA to AuNPs shows an alternative pathway for stabilizing the partially unfolded state of the protein and the resulting protein-nanocomposites shows prominent properties.29,30 However, the use of AuNPs may not only impart stabilization to the unfolded state of the protein but also can provide an optical label, while being water soluble and biologically benign. While the anticancer effect of the native α-LA is explored in the literature, its apo form (Ca2+ depleted) and the AuNPs stabilized by the apo-α-LA deserves further research.31 Therefore, in the present study, we focus on the green synthesis of luminescent AuNPs coated with bovine apo α-lactalbumin (apo-α-LA) as reducing and stabilizing agent at ambient conditions. The AuNPs thus generated using apo-α-LA (apo-α-LA-AuNPs) were characterized and demonstrated for their biocompatibility on L929 cells and anticancer activity on HeLa cells and breast cancer MCF-7 cells.Experimental
Apo-α-LA and HAuCl4·3H2O were procured from Sigma Aldrich Chem. Co., and used without further purification. Milli Q water was used for all the experiments. The apo-α-LA is a milk protein containing 123 amino acids with molecular weight of 14.2 kDa. All the eight cysteine residues present in apo-α-LA exists as four disulfide bridges besides possessing four tryptophans and three tyrosine residues.Preparation & separation of apo-α-LA-AuNPs
In order to make the protein coated AuNPs, a mixture of 10.8 mg of apo-α-LA (in 5 mL of water) and 2.4 mg of HAuCl4·3H2O (in 5 mL of water) was treated with 1 N NaOH (0.5 mL) that was added slowly over a period of 10 min as drop wise and the stirring was continued for several days. The formation of AuNPs resulted in brown colour. The solution of the AuNPs obtained after nine days was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 45 minutes and the supernatant was separated from the precipitate and the latter was dissolved in water for further analysis. The supernatant was subjected to absorption and TEM measurements and the precipitate was studied for ICPAES, spectroscopy, microscopy and the cell work.
000 rpm for 45 minutes and the supernatant was separated from the precipitate and the latter was dissolved in water for further analysis. The supernatant was subjected to absorption and TEM measurements and the precipitate was studied for ICPAES, spectroscopy, microscopy and the cell work.
Citrate-AuNPs
The citrate-AuNPs were prepared for the control studies. Nineteen mL of HAuCl4 was boiled and to this was added 1 mL of 1% trisodium citrate under vigorous stirring. After 15 min, the formation of citrate-AuNPs was observed as pinkish red colour solution.32Spectroscopy
UV-Visible absorption studies were performed on Varian instrument. One mL of sample was taken from the reaction mixture into a cuvette and the absorbance was measured at regular time intervals. Absorption spectra were recorded at a scan rate of 200 nm min−1, between 200 and 800 nm. The fluorescence spectra were measured on Perkin Elmer LS55 by exciting the solutions both at 280 and 365 nm. Other details are same as that used for the absorption spectra. Far-UV CD were recorded on JASCO-810 using quartz cuvette of 0.1 cm path length. CD spectra were accumulated at room temperature at a scan speed of 100 nm min−1 between 190–270 nm. Each time, 0.5 mL of sample was taken for the CD measurement. In order to confirm that the conformational changes were not affected by simple dilution, CD spectrum of apo-α-LA were recorded in water and found no changes indicating that the dilution has negligible effect.Microscopy
TEM experiments have been carried out on JEOL JEM 2100-F transmission electron microscope operating at 20–200 kV (resolution 2.4 Å). Carbon coated copper grids with 200 mesh have been used as substrate. A 20 μL of sample was drop casted on the TEM grid and allowed to dry under the IR lamp for 15 minutes. The ESEM experiments were carried out on JEOL JSM 7500 Field emission scanning electron microscope. The sample preparation is similar except for the use of carbon tape.ICPAES, XPS and DLS
For inductively coupled plasma atomic emission spectroscopy (ICPAES), one mL of sample from the stock solution was taken and diluted to 5 mL with water. The sample was kept for the dialysis for 24 h at RT by at least changing the water three times. The sample was analysed with ICPAES at 12 and 24 h. For X-ray photoelectron spectroscopy (XPS) study, 20 μL of sample was drop casted on the Al foil and allowed it to dry under the IR lamp for 30 minutes. Now the sample is ready for measuring its XPS spectrum on Kratos AXIS X-ray photoelectron spectrometer. The Dynamic light scattering (DLS) experiments were performed on Brookhaven 90Plus instrument. A 1 mL sample was diluted to 3 mL and the experiments were performed. Since DLS experiments are sensitive to the dust, the water was filtered through 0.2 μm filter paper.Cell work
Sulforhodamine-B (SRB) was performed to evaluate cell viability with L-929, HeLa and MCF7 cell lines. The cells were seeded into 96-well plates at densities of 1 × 104 cells per well for 24 h. Different concentrations of the suspension of samples were added to the cells and incubated for 24 h at 37 °C in the atmosphere of 5% CO2. Thereafter, the cells were washed thrice with phosphate buffer saline (PBS) and processed for SRB assay to determine the cell viability. For this, the cells were fixed with a solution of 50% trichloroacetic acid and stained with 0.4% SRB dissolved in 1% acetic acid. Cell-bound dye was extracted with 10 mM Tris buffer solution at pH = 10.5 and then the absorbance was measured at 560 nm using a plate reader. The cell viability was calculated as the ratio of the absorbance of the sample and the control and was expressed in %. The samples for the confocal laser scanning microscopy (CLSM) were prepared on the cover slip and air dried and were scanned under the laser source having the excitation of 365 nm using Olympus IX. The images were analyzed by Fluoview Viewer.Results and discussion
Synthesis and characterization of apo-α-LA-AuNPs
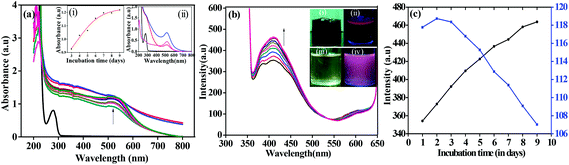
The apo-α-LA-AuNPs were synthesized by the treatment of apo-α-LA with the Au(III) solution under alkaline conditions as given in the Experimental. The formation of the AuNPs was followed by measuring the absorption spectra on different days and monitoring the SPR band formed at ∼515 nm (Fig. 1). The absorbance of the SPR band increases gradually supporting the nucleation followed by growth of AuNPs to have 10–15 nm size for the particles (inset (i) in Fig. 1a). The band observed at 280 nm in free protein is converted to a broad shoulder in AuNPs. Further, the absorbance of the 210 nm band dramatically decreases. All this supports the conjugation of the AuNPs to the protein to result in apo-α-LA-AuNPs. During the first two days, a broad feature with weak absorbance was observed in 450 to 550 nm indicating the formation of AuNCs. The stirring of the reaction mixture further resulted in a clear SPR band at ∼515 nm whose intensity grows over a period of time and saturates in about one week, suggesting the formation of stable AuNPs and this SPR corresponds to the AuNPs of 10–15 nm size.33The formation of AuNPs is slow because of the absence of any external chemical reducing agent, where the protein itself acts as reducing as well as stabilizing agent. All these features are evident from the absorption spectra shown in Fig. 1a. UV-Vis spectra were recorded for both the supernatant and the precipitate for the reaction mixtures obtained after 8 days, where the precipitate shows a very strong SPR band at ∼515 nm wherein the absorbance is at least three fold higher than that of the supernatant (inset (ii) in Fig. 1a). Both these fractions possesses band arising from the protein. As expected, the unbound protein was separated from apo-α-LA-AuNPs into the supernatant.
During the preparation of AuNPs, the reaction mixture was subjected to fluorescence emission study by exciting the solution at 280 as well as 340 nm. This was carried out for one week by measuring the spectra every day where the reaction mixture was stirred continuously. The emission intensity arising from the protein (λex = 280 nm) is quenched supporting that the protein is bound to AuNPs. The quenching of fluorescence intensity is attributed to the energy transfer from the donor aromatic residues (mainly tyrosine) to the surface of the acceptor AuNPs. However, the emission from the AuNPs observed at ∼410 nm arising from a 340 nm excitation increases as a function of time suggesting the formation of luminescent apo-α-LA-AuNPs as can be noticed from Fig. 1b. The deep brown colour solution exhibits red emission under UV light at 365 nm suggesting the formation of luminescent AuNPs. The emission spectra observed for apo-α-LA-AuNPs at ∼410 nm increases fluorescence intensity in its spectra as fluorescence intensity of the weak shoulder observed at 615 nm diminishes and this results in an isoemissive point centered at 535 nm (Fig. 1c), showing that the smaller nanoclusters formed initially converts into bigger AuNPs. As the protein structure is sensitive enough to the environmental effects, such as solvent, temperature, presence of metal ions, etc., one would expect changes in the secondary structure of the protein due to the development of AuNPs in the reaction. Indeed, loss of α-helix was observed in the CD spectra (Fig. 2a) during the synthesis of AuNPs. This is attributable to the breakage of the disulphide bonds in NaOH,34 and the resultant sulfhydral functions to involve in stabilizing the AuNPs. The shifts observed in the amide I, II and III bands, and the N–H & O–H stretching vibrations (Fig. 2b) are suggestive of the secondary structural changes in the protein upon capping the AuNPs.
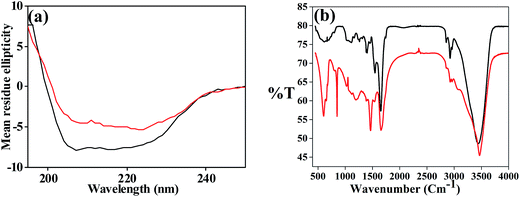 | ||
| Fig. 2 (a) CD and (b) FT-IR spectra. The black one is for apo-α-LA and the red one is for apo-α-LA-AuNPs. | ||
The existence of Au0 was supported by X-ray photo electron spectroscopy. The Au 4f7/2 appeared at 84.3 eV is assignable to the Au0 as can be seen from Fig. 3a. The 2p peak of S observed at 162.8 eV (Fig. 3b) supports the presence of thiolate binding (Au–S) on to the AuNPs. The EDAX performed on ESEM supports quantitatively the existence of Au0 and sulphur in the dialysed apo-α-LA-AuNPs. It also shows the presence of N, O and S along with the Au (Fig. 3c), supporting the presence of the protein in the AuNPs.
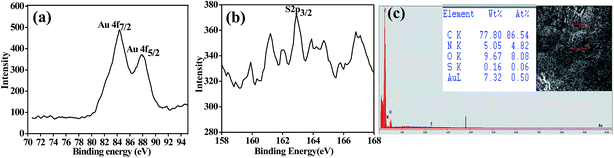 | ||
| Fig. 3 XPS spectra of apo-α-LA-AuNPs: (a) 4f for Au0 and (b) 2p for S. (c) ESEM of the dialysed samples of the apo-α-LA-AuNPs. Inset: elemental composition and the selected area for the EDAX. | ||
Microscopy characterization of apo-α-LA-AuNPs
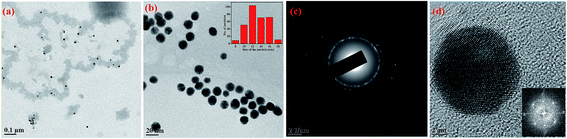
In order to confirm the features, such as, size, shape and crystallinity of the apo-α-LA-AuNPs formed, TEM studies were carried out. After 24 h of the reaction, the AuNPs formed were uneven in shape and size, and exhibit polydispersity, while the larger ones are spherical as can be seen from the micrographs given in Fig. 4. | ||
| Fig. 4 (a) to (d): TEM micrographs of apo-α-LA-AuNPs formed upon 24 hours of incubation at different scales. Inset in (d) is FFT of the corresponding image. | ||
The TEM micrographs that have been taken after the complete growth (one week), showed spherical AuNPs with size ranging from 10 to 16 nm. These AuNPs are protected by the protein shell as can be noticed from the contrast observed in TEM (Fig. 5a and b). The selected area electron diffraction (SAED) showed polycrystalline nature of the apo-α-LA-AuNPs and the HRTEM showed an interplanar distance of 0.231 nm as can be seen from Fig. 5c and d.
The TEM micrographs of the supernatant were abundant in protein though few apo-α-LA-AuNPs were in trace amounts. On the other hand, the precipitate showed good quantity of apo-α-LA-AuNPs (13 ± 3 nm) encapsulated in the protein as can be seen from the background contrast (Fig. 6a and b). In SAED the supernatant showed amorphous nature of the particles and that of the precipitate showed crystalline ones as can be seen from Fig. 6c. The TEM micrograph features noticed in Fig. 6d for the citrate-AuNPs and the corresponding absorption spectra shown in Fig. 6e, suggests that both the shape and size of the AuNPs coated with simple citrate and apo-α-LA are same.
In order to support the presence of protein shell capping the AuNPs, DLS studies were performed. DLS studies showed average hydrodynamic radius of 19.3 nm for apo-α-LA-AuNPs that can be observed from Fig. 7a, which is considerably higher than the average diameter of 13 ± 3 nm observed for the AuNPs in TEM. The size observed from the DLS is also greater than the size of the citrate-AuNPs from DLS (16.8 nm) as can be seen in Fig. 7b and that from TEM (16 ± 3 nm) further supporting the presence of protein shell in the AuNPs. In case of citrate-AuNPs there was no significant difference between the average hydrodynamic radius and average diameter from TEM, on the other hand, apo-α-LA-AuNPs showed greater difference in their diameters which is attributable to the presence of the protein shell on the AuNPs. In order to further support that the AuNPs were encapsulated into the protein, ICP-AES studies were performed after dialysis. The concentration of Au0 before dialysis was 16.7 ± 0.5 ppm and after the 24 h dialysis it is 14.4 ± 0.4 ppm. While, the dialysis removes the un-bound Au0 and un-reacted Au3+ (if any), the ICP-AES results suggest the encapsulation of AuNPs into the protein.
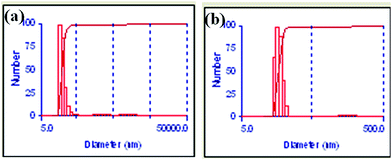 | ||
| Fig. 7 DLS data for, (a) apo-α-LA-AuNPs (mean diameter = 19.3 nm); (b) citrate-AuNPs (mean diameter = 16.8 nm). | ||
Biocompatibility of apo-α-LA-AuNPs
The biocompatibility of simple AuNPs with normal cell line is reported in the literature,35 however, no such reports were known for the apo-α-LA bound AuNPs. Therefore, the apo-α-LA-AuNPs thus reported in this paper were examined for their biocompatibility with the normal mouse fibroblast cells (L929) by performing the SRB assay. In the concentration range of 2 to 8 μM, the apo-α-LA-AuNPs showed cell viability of 97–98% (Fig. 8) suggesting the non-toxic nature of these apo-α-LA coated AuNPs towards healthy cells and hence these are biocompatible.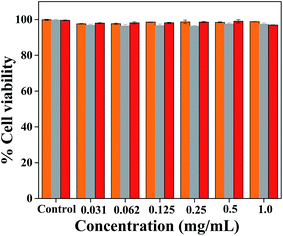 | ||
| Fig. 8 Bar diagram for cell viability vs. concentration for L929 cells treated with, citrate-AuNPs (orange yellow), free apo-α-LA (grey) and apo-α-LA-AuNPs (red). | ||
Anticancer activity of apo-α-LA-AuNPs on HeLa cells and MCF-7 cells
The native form of the protein (α-LA) is known to have minimal anti cancer effect, however, shows enhanced anticancer effect when it undergoes conformational change to result in BAMLET which are stabilised by oleic acid.26,27,31 Therefore, herein, we explore the anticancer activity of apo-α-LA-AuNPs on HeLa (cervical cancer cells) and MCF-7 (breast cancer cells). The results were compared with appropriate controls, viz., free apo-α-LA and citrate-AuNPs. It was observed that apo-α-LA-AuNPs have shown effect on both the HeLa cells and MCF-7 cells as shown in the Fig. 9. In the concentration range 2–8 μM, the apo-α-LA-AuNPs brings cell death of ∼25–35% in case of HeLa cells, which is only marginally higher than the control, i.e., free apo-α-LA that causes cell death to an extent of 10–20%. On the other hand, the apo-α-LA-AuNPs causes cell death of 70–75% in the same concentration range in case of MCF-7 cells, which is far greater than the free protein control (10–20%) as well as the citrate-AuNPs control (17–23%). The study clearly suggests that the apo-α-LA-AuNPs are capable of killing breast cancer cells by >200% as compared to the cervical cancer cells.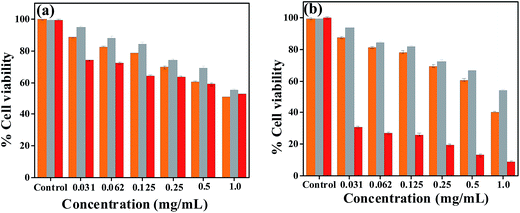 | ||
| Fig. 9 Cell viability bar diagrams for, (a) HeLa cells and (b) MCF-7 cells. Citrate-AuNPs (orange yellow), free apo-α-LA (grey) and apo-α-LA-AuNPs (red). | ||
Microscopy of apo-α-LA-AuNPs treated MCF7 and HeLa cells
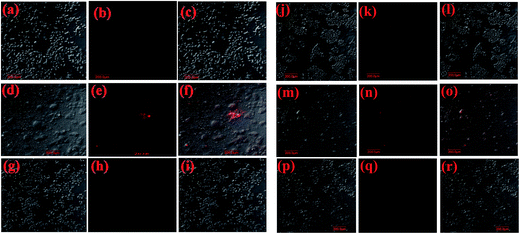
Confocal microscopy studies were carried out using the luminescence property (λex = 365 nm) of the apo-α-LA-AuNPs as reported in this paper (Fig. 10).This study showed that the MCF-7 cells treated with apo-α-LA-AuNPs were killed to a large extent, while those treated with free apo-α-LA or citrate-AuNPs were killed to a smaller extent and does not exhibit any luminescence at this excitation. Significant morphological changes were noticed when the MCF-7 cells treated with the apo-α-LA-AuNPs due to apoptosis and luminescence is noticed supporting the entry of these NPs inside the cells (Fig. 10d–f). However, no such morphological changes were observed when these cells were treated with either free apo-α-LA or citrate-AuNPs and no fluorescence was observed. Comparison of the data obtained in case of MCF-7 (Fig. 10a–i) cells with that obtained from HeLa cells (Fig. 10j–r) clearly suggests that the apo-α-LA-AuNPs specifically target and kill the breast cancer cells to a greater extent as compared to the cervical ones.
Conclusions and comparisons
The AuNPs coated with apo-α-LA (viz., apo-α-LA-AuNPs) were synthesized for the first time by using the apo-α-LA protein alone, without the use of any external reducing and or capping agent. Thus in the present case, the protein acts as both reducing as well as capping agent. While the apo-α-LA protein reduces Au3+ to Au0 using the side chain moieties of Tyr, the side chains of Cys will assist in stabilizing the nanoparticles formed by capping through the sulfhydral functions and thus represents a green synthesis.The AuNPs thus formed through the green synthesis method were characterized by different analytical, spectral and microscopy methods. The absorption spectra provided an SPR signature for the formation of apo-α-LA-AuNPs at 515 nm that corresponds to ∼15 nm size particles where the breadth of the peak supports polydispersity. The additional peak observed at ∼280 nm supports the presence of protein coating on these AuNPs. Strong fluorescence emission observed at 615 nm upon excitation at 340 nm supports the luminescent character of the apo-α-LA-AuNPs. Thus the optically labelled apo-α-LA-AuNPs are useful in cell imaging studies. The CD spectra clearly demonstrated the secondary structural changes that occurred in the protein upon the formation of AuNPs followed by capping. Thus the AuNPs supported the stabilization of a conformationally different protein and hence the apo-α-LA-AuNPs thus formed are of great relevance to study their anticancer activity, since BAMLET also exhibit weak antiproliferation activity. While the reaction mixture showed particles in the range 9–16 nm size in TEM, the dialysed samples showed 10–16 nm size. Comparison of the particle size observed from TEM with that observed from the hydrodynamic radius from DLS study (19.3 nm) supports the protein coating on the nanoparticles. The presence of the protein shell is also evident from TEM micrograph that shows lower contrast image for the protein. The analytical results obtained from ICPAES and ESEM clearly supported the presence of gold and the protein in the NPs formed. The presence of Au0 was further confirmed by XPS by observing a peak at 84.3 eV (Au 4f7/2) and sulphur from the protein at 162.8 eV (S 2p3/2).
The cell viability studies carried out using healthy cells, viz., fibroblast cells (L929), showed almost 100% survival and hence the apo-α-LA-AuNPs are non-toxic to normal cells. Since these apo-α-LA-AuNPs have capping by the conformationally altered protein (apo-α-LA), the toxicity of these AuNPs on the cancer cells was studied. The results showed ∼75% of killing in case of MCF-7 cells and only ∼30% of killing in case of HeLa cells and hence all this clearly suggests that the apo-α-LA-AuNPs are more selective towards destroying the breast cancer cells over the cervical ones. The conformationally altered and AuNP bound apo-α-LA thus showed ∼400% increase in its anti cancer activity as compared to the simple apo-α-LA. Thus the luminescent apo-α-LA-AuNPs play influential role in cancer therapy in general and on the breast cancer cells in particular.
Acknowledgements
CPR thanks the DST (SERB & Nano Mission), CSIR and BRNS for financial support. YSD acknowledge UGC and SD acknowledge IIT Bombay for their fellowships. We acknowledge SAIF, IIT Bombay for instrumental facility and Department of Physics for SPM and XPS facility.References
- B. M. Kim, M. J. Hackett, J. Park and T. Hyeon, Chem. Mater., 2014, 26, 59–71 CrossRef CAS.
- S. Guo and E. Wang, Nano Today, 2011, 6, 240–264 CrossRef CAS PubMed.
- A. Tomar and G. Garg, Global J. Pharmacol., 2013, 7, 34–38 Search PubMed.
- X. Liu, M. Atwater, J. Wang and Q. Huo, Colloids Surf., A, 2007, 58, 3–7 CAS.
- E. Biosselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759–1782 RSC.
- R. A. Sperling, P. R. Gil, F. Zhang, M. Zanella and W. J. Parak, Chem. Soc. Rev., 2008, 37, 1896–1908 RSC.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- P. K. Jain, K. S. Lee, I. H. El-Sayed and M. A. El-Sayed, J. Phys. Chem. B, 2006, 110, 7238–7248 CrossRef CAS PubMed.
- T. Kong, J. Zeng, X. Wang, X. Yang, J. Yang, S. McQuarrie, A. McEwan, W. Roa, J. Chen and J. Z. Xing, Small, 2008, 4, 1537–1543 CrossRef CAS PubMed.
- H. Chen, X. Kou, Z. Yang, W. Ni and J. Wang, Langmuir, 2008, 24, 5233–5237 CrossRef CAS PubMed.
- D. Choudhury, P. L. Xavier, K. Chaudhari, R. John, A. K. Dasgupta, T. Pradeep and G. Chakrabarti, Nanoscale, 2013, 5, 4476–4489 RSC.
- K. Lee and M. A. El-Sayed, J. Phys. Chem. B, 2006, 110, 19220–19225 CrossRef CAS PubMed.
- P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Plasmonics, 2007, 2, 107–118 CrossRef CAS.
- C. Huang, Z. Yang, K. Lee and H. Chang, Angew. Chem., 2007, 119, 6948–6952 CrossRef.
- E. Oh, M. Hong, D. Lee, S. Nam, H. C. Yoon and H. Kim, J. Am. Chem. Soc., 2005, 127, 3270–3271 CrossRef CAS PubMed.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389–1392 CrossRef CAS.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC.
- T. K. Sau and C. J. Murphy, J. Am. Chem. Soc., 2004, 126, 8648–8649 CrossRef CAS PubMed.
- S. P. Dubey, M. Lahtinen and M. Sillanpaa, Colloids Surf., A, 2010, 364, 34–41 CrossRef CAS PubMed.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940–13941 CrossRef CAS PubMed.
- A. Baksi, P. L. Xavier, K. Chaudhari, N. Goswami, S. K. Pal and T. Pradeep, Nanoscale, 2013, 5, 2009–2016 RSC.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- S. L. Smitha, D. Philip and K. G. Gopchandran, Spectrochim. Acta, Part A, 2009, 74, 735–739 CrossRef CAS PubMed.
- D. Philip, Spectrochim. Acta, Part A, 2009, 73, 650–653 CrossRef PubMed.
- L. Gustafsson, O. Hailgren, A. Mossberg, J. Pettersson, W. Fischer, A. Aronsson and C. Svanborg, J. Nutr., 2005, 135, 1299–1303 CAS.
- M. Svensson, J. Fast, A. Mossberg, C. Duringer, L. Gustafsson, O. Hallgren, C. L. Brooks, L. Berliner, S. Linse and C. Svanborg, Protein Sci., 2003, 12, 2794–2804 CrossRef CAS PubMed.
- M. Svensson, A. Hakansson, A. Mossberg, S. Linset and C. Svanborg, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 4221–4226 CrossRef CAS.
- T. Kamijima, A. Ohmura, T. Sato, K. Akimoto, M. Itabashi, M. Mizuguchi, M. Kamiya, T. Kikukawa, T. Aizawa, M. Takahashi, K. Kawano and M. Demura, Biochem. Biophys. Res. Commun., 2008, 376, 211–214 CrossRef CAS PubMed.
- S. M. Lystvet, S. Volden, G. Singh, M. Yasuda, Q. Halskau and W. R. Golmm, RSC Adv., 2013, 203(3), 482–495 RSC.
- S. M. Lystvet, S. Volden, Q. Halskau and W. R. Golmm, Soft Matter, 2011, 7, 11501–11509 RSC.
- S. M. Lystvet, S. Volden, G. Singh, I. M. Rundgren, H. Wen, Q. Halskau and W. R. Golmm, J. Phys. Chem. C, 2013, 117, 2230–2238 CAS.
- J. Turkevicphe, P. C. Stevenson and J. Hillier, Faraday Soc., 1951, 11, 55–75 RSC.
- D. Ghosh, D. Sarkar, A. Girigoswami and N. Chattopadhyay, J. Nanosci. Nanotechnol., 2010, 10, 1–6 CrossRef PubMed.
- T. M. Florence, Biochem. J., 1980, 189, 507–520 CAS.
- C. J. Murphy, A. M. Gole, J. W. Stone, P. N. Sisco, A. M. Alkilany, E. C. Goldsmith and S. C. Baxter, Acc. Chem. Res., 2008, 41, 1721–1730 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |