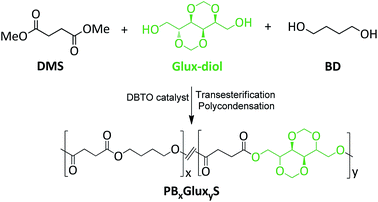
Bio-based PBS copolyesters derived from a bicyclic D-glucitol†
Elena Zakharova,
Abdelilah Alla,
Antxon Martínez de Ilarduya and
Sebastián Muñoz-Guerra*
Departament d'Enginyeria Química, Universitat Politècnica de Catalunya, ETSEIB, Diagonal 647, 08028 Barcelona, Spain. E-mail: sebastian.munoz@upc.edu
First published on 12th May 2015
Abstract
2,4:3,5-di-O-methylene-D-glucitol (Glux-diol) was used for the synthesis of poly(butylene succinate) (PBS) copolyesters by melt polycondensation. Glux-diol possess a rigid bicyclic asymmetric structure made of two fused 1,3-dioxane rings and two hydroxyl functions at the end positions. Copolyesters were prepared over the whole range of compositions with molecular weights varying from 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 46
000 to 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a random microstructure. The thermal stability of PBS did not significantly alter with the presence of Glux units. The glass transition temperatures (Tg) steadily increased from −28 to 80 °C along the whole copolyester series with the insertion of Glux. On the contrary, melting temperature (Tm) and crystallinity decreased because of the lack of regularity of the polymer chain although copolyesters with contents of Glux units up to 30 mole% were semicrystalline. The stress–strain behavior changed according to variations produced in thermal transitions. The replacement of 1,4-butanediol by Glux-diol slightly increased both the hydrolytic degradability and the biodegradability of PBS. Compared to other bicyclic sugar-based diols reported in the literature, Glux-diol appeared to be more efficient in both increasing the Tg and enhancing the susceptibility to hydrolysis of PBS.
000 g mol−1 and a random microstructure. The thermal stability of PBS did not significantly alter with the presence of Glux units. The glass transition temperatures (Tg) steadily increased from −28 to 80 °C along the whole copolyester series with the insertion of Glux. On the contrary, melting temperature (Tm) and crystallinity decreased because of the lack of regularity of the polymer chain although copolyesters with contents of Glux units up to 30 mole% were semicrystalline. The stress–strain behavior changed according to variations produced in thermal transitions. The replacement of 1,4-butanediol by Glux-diol slightly increased both the hydrolytic degradability and the biodegradability of PBS. Compared to other bicyclic sugar-based diols reported in the literature, Glux-diol appeared to be more efficient in both increasing the Tg and enhancing the susceptibility to hydrolysis of PBS.
Introduction
A growing interest for chemicals derived from renewable resources which are able to replace oil-based monomers for the production of industrial polymers is noticeable in these last years.1,2 Additionally, environmental pollution has recently become a big problem with both social and technical repercussions due largely to the high impact of plastic wastes.3 The most popular approach followed today to respond to these concerns is to replace the commodity synthetic polymers with bio-based polymers.4–6 In fact, polymers made from monomers derived from non-fossil materials are sustainable and also totally or partially susceptible to microbial degradation.7 Aliphatic polyesters such as poly(L-lactic acid), poly(butylene succinate), and polyhydroxyalkanoates among others, constitute primary examples of bio-based polymers that distinguish by being fully renewable and displaying partial or total biodegradability. Such features make aliphatic polyesters especially suitable for large-tonnage applications where large consumption of raw materials and high environmental impact are major concerns, e.g. packaging, disposable items and agricultural mulch films.8Poly(butylene succinate) (PBS) is one of the members of the aliphatic polyester family that is receiving greatest attention. This polyester not only may be built by using exclusively renewable feedstock but it also displays mechanical properties comparable to other extensively used conventional polymers.9 Furthermore, PBS has been demonstrated to exhibit significant biodegradation in soil, activated sludge and sea water.10 Due to its outstanding potential, PBS is today in the focus of an intensive research addressed to improve its thermal and mechanical properties without significant detriment to its sustainability and biodegradability. Copolymerization involving cyclic comonomers and blending with nanofillers are the main approaches followed in this regard.11,12
Carbohydrates stand out as very convenient raw materials for furnishing polycondensation monomers. They are relatively inexpensive, readily available, and provide broad functional diversity. In recent years, a large number of examples of polycondensation polymers made from carbohydrate derivatives have been reported in the literature.13,14 Cyclic carbohydrate-based monomers are particularly relevant because their stiff structures are able to increase the glass transition temperature and hence to improve certain polymer properties such as heat deflection temperature, hardness, tearing resistance and permeability. Isohexides and more specifically isosorbide, are bicyclic dianhydride diols coming from hexoses that have been widely investigated for their potential to enhance the performance of both aliphatic and aromatic polyesters.15,16 More recently, carbohydrate-based bicyclic diols and diacids with a diacetal constitution have emerged as a new class of bio-based monomers with a potential at least comparable to that of isohexides.17,18 Most exciting results have been those attained with aromatic copolyesters containing fused diacetalized bicyclic units derived from D-mannose and D-glucose.19,20 These novel sugar-based copolyesters have been reported to exhibit enhanced thermal properties and biodegradability when compared to PET and PBT.19b,20b
The purpose of this work is to explore the effects on properties of PBS caused by the presence of carbohydrate-based diacetalized bicyclic units in the polymer chain, more specifically of 2,4:3,5-di-O-methylene-D-glucitol, abbreviated as Glux-diol. We have very recently reported on PBS copolyesters made from Manx-diol, the stereoisomer of Glux-diol that derives from D-mannose.21 Both isomers consist of two fused 1,3-dioxane rings structure sharing a C6-segment backbone that bears two hydroxyl functions at the end positions. At difference with Manx-diol, Glux-diol is asymmetric so its two OH groups are spatially and hence chemically different (Scheme 1). Random PBS copolyesters containing Manx units could be obtained with Mw above 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, they were semicrystalline for the whole range of compositions and displayed enhanced Tg and biodegradability. Since polymerization rate as well as polymer properties are largely depending on monomer symmetry, it is of much conceptual interest to compare Manx and Glux diols as comonomers for the production of PBS copolyesters. Additionally, data obtained from this study can be related to those reported for PBS copolyesters containing isosorbide in order to assess diacetalized and dianhydride bicyclic diols as optional comonomers for their capacity to improve PBS properties. The study is also of practical relevance since Glux-diol is a compound coming from D-glucose, the most available monosaccharide in nature.
000, they were semicrystalline for the whole range of compositions and displayed enhanced Tg and biodegradability. Since polymerization rate as well as polymer properties are largely depending on monomer symmetry, it is of much conceptual interest to compare Manx and Glux diols as comonomers for the production of PBS copolyesters. Additionally, data obtained from this study can be related to those reported for PBS copolyesters containing isosorbide in order to assess diacetalized and dianhydride bicyclic diols as optional comonomers for their capacity to improve PBS properties. The study is also of practical relevance since Glux-diol is a compound coming from D-glucose, the most available monosaccharide in nature.
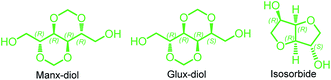 | ||
| Scheme 1 Chemical structures of sugar-based bicyclic diols with specification of their respective stereocenters. | ||
Experimental part
Materials
The reagents 1,4-butanediol (BD) (97%), dimethyl succinate (DMS) (>99%), 1,5-D-gluconolactone (99%), lithium aluminium hydride (95%), paraformaldehyde (>95%), sodium hydroxide (>97%) and the catalyst dibutyl tin oxide (DBTO, 98%), were purchased from Sigma-Aldrich. Lipase from porcine pancreas (activity 15–35 U, 3 mg−1, pH 8.0, 37 °C) was also purchased from Sigma-Aldrich. Solvents used for purification, synthesis and characterization were all of either technical or high-purity grade and they were purchased from Panreac and used as received without further purification. Irganox 1010, Irgafos 126 antioxidants were a generous gift from BASF. The cyclic diol 2,4:3,5-di-O-methylene-D-glucitol (Glux-diol) has been prepared following a procedure well described in the recent literature.22General methods
1H and 13C NMR spectra were recorded on a Bruker AMX-300 spectrometer at 25.0 °C operating at 300.1 and 75.5 MHz, respectively. Polyesters were dissolved either in deuterated chloroform or in a mixture of deuterated chloroform/trifluoroacetic acid (TFA) (1/1), and spectra were internally referenced to tetramethylsilane (TMS). About 10 and 50 mg of sample dissolved in 1 mL of solvent were used for 1H and 13C NMR, respectively. Sixty-four scans were acquired for 1H and 1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for 13C with 32 and 64 K data points as well as relaxation delays of 1 and 2 s, respectively. Viscosities of polyesters were measured in dichloroacetic acid at 25.00 ± 0.01 °C, using a capillary viscosimeter at concentrations ranging from 5 to 10 mg mL−1. Gel permeation chromatograms were acquired at 35.0 °C with a Waters equipment provided with a refraction-index detector. The samples were chromatographed with 0.05 M sodium trifluoroacetate-hexafluoroisopropanol (NaTFA-HFIP) using a PL HFIPgel 300 × 7.5 mm column with a flow rate of 0.5 mL min−1. Chromatograms were calibrated against poly(methyl methacrylate) (PMMA) monodisperse standards. The thermal behavior of polyesters was examined by DSC using a Perkin Elmer DSC Pyris 1. DSC data were obtained from 3 to 5 mg samples at heating/cooling rates of 10 °C min−1 under a nitrogen flow of 20 mL min−1. Indium and zinc were used as standards for temperature and enthalpy calibration. The glass-transition temperatures were determined by the tangent method at a heating rate of 20 °C min−1 from rapidly melt-quenched polymer samples. The treatment of samples for isothermal crystallization experiments was the following: the thermal history was removed by heating the sample up to 200 °C and left at this temperature for 5 min, and then it was cooled at 20 °C min−1 to the selected crystallization temperature, where it was left to crystallize until saturation. Thermogravimetric analyses were performed under a nitrogen flow of 20 mL min−1 at heating rate of 10 °C min−1, within a temperature range of 30 to 600 °C, using a Mettler Toledo TGA/DSC 1 thermobalance. Sample weights of about 10–15 mg were used in these experiments. Films for mechanical properties with a thickness of ∼200 μm were prepared by the hot-pressing method. The tested samples were cut into strips with a width of 3 mm while the distance between testing marks was 10 mm. The tensile strength, elongation at break and Young's modulus were measured at a stretching rate of 30 mm min−1 on a Zwick 2.5/TN1S testing machine coupled with a compressor Dalbe DR 150. Each sample was measured five times. X-ray diffraction patterns were recorded on the PANalytical X'Pert PRO MPD θ/θ diffractometer using the Cu Kα radiation of wavelength 0.1542 nm from powdered samples coming from synthesis.
000 for 13C with 32 and 64 K data points as well as relaxation delays of 1 and 2 s, respectively. Viscosities of polyesters were measured in dichloroacetic acid at 25.00 ± 0.01 °C, using a capillary viscosimeter at concentrations ranging from 5 to 10 mg mL−1. Gel permeation chromatograms were acquired at 35.0 °C with a Waters equipment provided with a refraction-index detector. The samples were chromatographed with 0.05 M sodium trifluoroacetate-hexafluoroisopropanol (NaTFA-HFIP) using a PL HFIPgel 300 × 7.5 mm column with a flow rate of 0.5 mL min−1. Chromatograms were calibrated against poly(methyl methacrylate) (PMMA) monodisperse standards. The thermal behavior of polyesters was examined by DSC using a Perkin Elmer DSC Pyris 1. DSC data were obtained from 3 to 5 mg samples at heating/cooling rates of 10 °C min−1 under a nitrogen flow of 20 mL min−1. Indium and zinc were used as standards for temperature and enthalpy calibration. The glass-transition temperatures were determined by the tangent method at a heating rate of 20 °C min−1 from rapidly melt-quenched polymer samples. The treatment of samples for isothermal crystallization experiments was the following: the thermal history was removed by heating the sample up to 200 °C and left at this temperature for 5 min, and then it was cooled at 20 °C min−1 to the selected crystallization temperature, where it was left to crystallize until saturation. Thermogravimetric analyses were performed under a nitrogen flow of 20 mL min−1 at heating rate of 10 °C min−1, within a temperature range of 30 to 600 °C, using a Mettler Toledo TGA/DSC 1 thermobalance. Sample weights of about 10–15 mg were used in these experiments. Films for mechanical properties with a thickness of ∼200 μm were prepared by the hot-pressing method. The tested samples were cut into strips with a width of 3 mm while the distance between testing marks was 10 mm. The tensile strength, elongation at break and Young's modulus were measured at a stretching rate of 30 mm min−1 on a Zwick 2.5/TN1S testing machine coupled with a compressor Dalbe DR 150. Each sample was measured five times. X-ray diffraction patterns were recorded on the PANalytical X'Pert PRO MPD θ/θ diffractometer using the Cu Kα radiation of wavelength 0.1542 nm from powdered samples coming from synthesis.
Polymer synthesis
Copolyesters of PBS containing Glux units (PBxGluxyS with subscripts x and y standing for mole% of BD and Glux units in the feed respectively) were synthesized by reaction of dimethyl succinate with mixtures of 1,4-butanediol and 2,4:3,5-di-O-methylene-D-glucitol at different selected ratios. PBS and PGluxS homopolyesters were obtained by polycondensation of dimethyl succinate with 1,4-butanediol and 2,4:3,5-di-O-methylene-D-glucitol, respectively. Since diols were partially streamed off by the nitrogen flow and also volatilized when the high vacuum was applied, a mole-10% excess of the diols respect to the diester monomer was used in all cases. The antioxidants Irganox 1010 (0.2% w/w) and Irgafos 126 (0.6% w/w) were added to minimize degradation of thermally sensitive sugar-based monomers. The same reaction protocol was applied for all compositions. Reactions were carried out in a three-necked, cylindrical bottom flask equipped with a mechanical stirrer, a nitrogen inlet and a vacuum distillation outlet. The reactants were stirred to get a homogeneous mixture and DBTO (0.4–0.6 mole% respect to the total of monomers) was added as catalyst. The transesterification step was performed for 3–5 h at 160 °C under nitrogen flow, and polycondensation for 7–8 h at 160–180 °C under vacuum (0.03–0.06 mbar). The final reaction mixture was cooled to room temperature under a nitrogen flow to prevent degradation, the resulting solid mass was dissolved in chloroform, and the polymer precipitated with methanol, collected by filtration and dried under vacuum. The NMR data ascertaining their constitution and purity are described below.Hydrolytic degradation and biodegradation
Films for hydrolytic degradation and biodegradation studies were prepared with a thickness of ∼200 μm by casting from chloroform solution at a polymer concentration of 100 g L−1. The films were cut into 10 mm diameter, 20–30 mg weight disks and dried under vacuum to constant weight. For hydrolytic degradation, samples were immersed in vials containing 10 mL of either citric acid buffer pH 2.0 or sodium phosphate buffer pH 7.4 at 37 °C. The enzymatic degradation was carried out at 37 °C in vials containing 10 mL of a pH 7.4 buffered sodium phosphate solution with added lipase from porcine pancreas (10 mg) and replacing the supernatant every 72 h to maintain the enzyme activity. In both cases, the disks were withdrawn from the incubation medium after scheduled periods of time, washed carefully with distilled water, dried to constant weight, and analyzed by GPC chromatography and NMR spectroscopy.Results and discussion
Synthesis and chemical structure
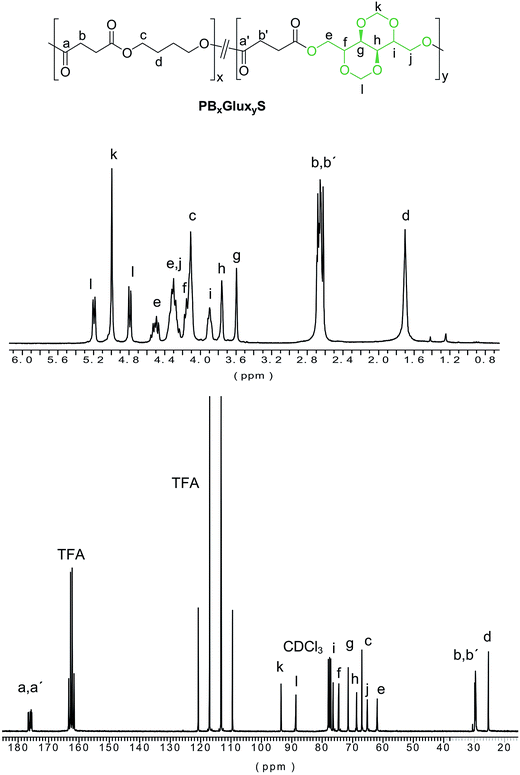
The monomer 2,4:3,5-di-O-methylene-D-glucitol (Glux-diol) with the required purity and in satisfactory yield was prepared from commercially available 1,5-D-gluconolactone as it has been previously reported by us.22 Polycondensation in the melt was the method chosen to prepare both homopolyesters and copolyesters in agreement with that is usual in the industrial practice, and the applied procedure conditions were as close as possible to those reported for the synthesis of Manx-containing PBS copolyesters.21 As it is depicted in Scheme 2, the polymerization procedure consisted in two steps, first generation of hydroxyl capped oligoesters by transesterification under a nitrogen flow to prevent decomposition of Glux, and second, polycondensation under vacuum to remove the excess of BD as much as possible. Reaction conditions regarding time and temperature were optimized for each individual case. Both the homopolyester PGluxS and the series of copolyesters PBxGluxyS containing Glux units from 5 up to 70 mole% were thus synthesized.The chemical constitution of the polyesters was assessed by NMR. As an example, both 1H and 13C NMR spectra of PB50Glux50S with indication of all signals assignments are shown in Fig. 1. NMR spectra of PB90Glux10S and PGluxS are provided in the ESI file (Fig. SI-1 and SI-2†). Data regarding composition, molecular weight and microstructure of PBxGluxyS copolyesters and homopolyesters are collected in Table 1. Copolyester compositions were determined by integration of the proton signals arising from BD and Glux units in the by 1H NMR spectra. As it is seen in Table 1, copolyester compositions are very close to those used in their respective feeds with a slight excess in the Glux content. The GPC analysis revealed that polyesters were obtained with weight average molecular weights within the 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–26
000–26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 range with molar-mass dispersities oscillating between 2.2 and 3.1. The general trend is that molecular weights slightly decrease with the increasing amount of Glux units in the polymer chain so that the minimum value is attained for the PGluxS homopolyester. Intrinsic viscosities decreased from 1.0 to near 0.4 dL g−1 in agreement with the trend observed for molecular weights. According to which has been repeatedly noticed for other polyesters containing sugar residues,19 such a trend is very likely determined by the high sensitivity to heat of 2,4:3,5-di-O-methylene-D-glucitol and the relatively high temperatures used in the polymerization reaction.
000 range with molar-mass dispersities oscillating between 2.2 and 3.1. The general trend is that molecular weights slightly decrease with the increasing amount of Glux units in the polymer chain so that the minimum value is attained for the PGluxS homopolyester. Intrinsic viscosities decreased from 1.0 to near 0.4 dL g−1 in agreement with the trend observed for molecular weights. According to which has been repeatedly noticed for other polyesters containing sugar residues,19 such a trend is very likely determined by the high sensitivity to heat of 2,4:3,5-di-O-methylene-D-glucitol and the relatively high temperatures used in the polymerization reaction.
| Polyester | Composition (mol mol−1) | Molecular weight | Microstructure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Feed | Copolyestera | [η]b (dL g−1) | Mnc | Mwc | Đc | Average sequence length | Rd | ||
| XBD/XGlux | nB | nG | |||||||
| a Molar composition determined by integration of 1H NMR spectra.b Intrinsic viscosity measured in dichloroacetic acid at 25 °C.c Determined by GPC in HFIP against PMMA standards.d Degree of randomness of copolyesters calculated on the basis of the 13C NMR analysis. | |||||||||
| PBS | 100/0 | 100/0 | 1.00 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
2.5 | — | — | — |
| PB95Glux5S | 95/5 | 94.4/5.6 | 0.71 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.6 | 9.9 | 1 | 1.10 |
| PB90Glux10S | 90/10 | 88.9/11.1 | 0.65 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.9 | 6.3 | 1.2 | 0.96 |
| PB70Glux30S | 70/30 | 71.2/28.8 | 0.60 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.8 | 2.6 | 1.7 | 0.98 |
| PB50Glux50S | 50/50 | 46.2/53.8 | 0.59 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.8 | 1.6 | 2.6 | 1.00 |
| PB30Glux70S | 30/70 | 25.4/74.6 | 0.60 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
3.1 | 1.3 | 4.8 | 0.98 |
| PGluxS | 0/100 | 0/100 | 0.41 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.2 | — | — | — |
The microstructure of the copolyesters was determined by 13C NMR taking benefit from the sensitiveness of the carbonyl groups to the sequence distribution at the dyads level (BB, BG, GB, GG). As a consequence of the occurrence of different dyads and also of the two orientations for the Glux unit, the CO signal splits into multiple peaks that appear spread within the 176.8–175.3 ppm interval (Fig. 2). Nevertheless, three groups of peaks may be discerned in such spectra which are arising from the four types of diol-dyads present in the copolyester chain. Although it is known that different carbons frequently have different relaxation times, it is not the case because the composition calculated using these carbon signals was the same as that obtained by 1H NMR. Then, the integration of all the dyad-associated peaks and application of the equations given below, allowed estimating the number average sequence lengths to evaluate the microstructure of the copolyesters according to the degree of randomness R.
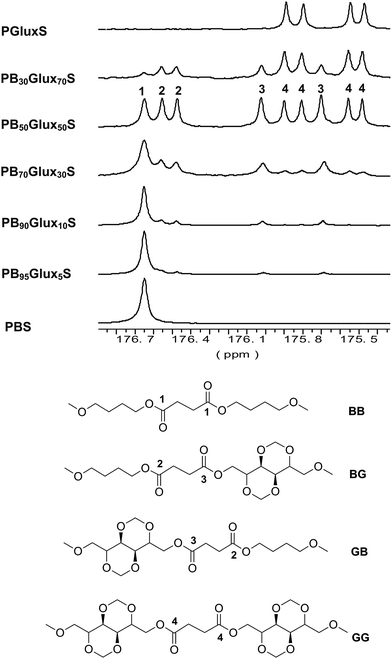 | ||
| Fig. 2 13C NMR spectra showing the changes undergone by the carbonyl signal of PBxGluxyS copolyesters with variations in composition. | ||
The values resulting from these calculations are given in Table 1 and they indicate that an almost random microstructure is shared by all the copolyesters (R ∼ 1).
| nB = (BB + 0.5(BG + GB))/0.5(BG + GB) |
| nG = (GG + 0.5(GB + BG))/0.5(BG + GB) |
| R = 1/nB + 1/nG |
Thermal properties and crystallization
The basic thermal properties of the obtained copolyesters were evaluated by TGA and DSC with special attention to the influence of the presence of the Glux units on decomposition, melting and glass transition temperatures. Data afforded by these analyses are collected in Table 2.| Polyester | TGAa | DSCb | XRDc | Stress–strain essaysd | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First heating | Cooling | Second heating | t1/2 (min) (at °C) | d (nm) | E (MPa) | σmax (MPa) | ε (%) | |||||||||||
| oTd (°C) | maxTd (°C) | RW (%) | Tg (°C) | Tm (°C) | ΔHm (J g−1) | Tc (°C) | Tc (°C) | Tm (°C) | ΔHm (J g−1) | (75) | (80) | (85) | (90) | |||||
| a Onset decomposition temperature corresponding to 5% of weight loss (oTd), temperature for maximum degradation rate (maxTd), and % of weight remaining after heating at 600 °C (RW).b Glass-transition temperature (Tg) taken as the inflection point of the heating DSC traces of melt-quenched samples recorded at 20 °C min−1. Melting (Tm) and crystallization (Tc) temperatures, and melting enthalpy (ΔHm) measured at heating/cooling rates of 10 °C min−1. Isothermal crystallization half-time (t1/2) determined at the indicated temperatures.c Bragg spacings measured by powder X-ray diffraction.d Elastic modulus (E), maximum stress (σ) and elongation to break (ε) measured by tensile testing from hot-pressing films. | ||||||||||||||||||
| PBS | 340 | 396 | 3 | −37 | 113 | 78 | 78 | 99 | 114 | 62 | — | — | 1.9 | 5.3 | 0.45, 0.40, 0.39 | 545 ± 11 | 32 ± 2 | 9 ± 1 |
| PB95Glux5S | 332 | 390 | 5 | −28 | 106 | 70 | 62 | 90 | 106 | 63 | 1.2 | 2.3 | — | — | 0.45, 0.40, 0.39 | 482 ± 10 | 13 ± 2 | 4 ± 1 |
| PB90Glux10S | 330 | 392 | 3 | −20 | 96 | 54 | 33 | 30 | 96 | 48 | 18.7 | 28.0 | — | — | 0.45, 0.40, 0.39 | 370 ± 13 | 16 ± 1 | 10 ± 2 |
| PB70Glux30S | 337 | 405 | 7 | 14 | 59 | 33 | — | — | — | 0.46, 0.40 | 348 ± 15 | 11 ± 1 | 4 ± 2 | |||||
| PB50Glux50S | 343 | 407 | 9 | 54 | — | — | — | — | — | 1093 ± 16 | 15 ± 2 | 3 ± 2 | ||||||
| PB30Glux70S | 338 | 403 | 8 | 80 | — | — | — | — | — | 1356 ± 19 | 40 ± 3 | 6 ± 2 | ||||||
| PGluxS | 264 | 403 | 9 | 103 | — | — | — | — | — | — | — | — | ||||||
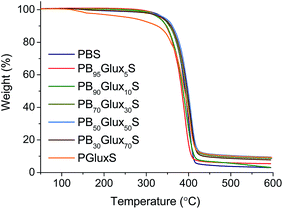
The recorded TGA traces for the whole series are compared in Fig. 3. All traces except that of PGluxS obey the same behavior pattern consisting of one only decomposition step that starts around 340 °C, fells down at the proximities of 400 °C and leaves less than 10% (w/w) of residual weight (see Fig. SI-3†). A detailed comparison of the decomposition parameters reveals that the insertion of the Glux units in PBS does not alter significantly the thermal stability of the parent polyester provided that the case for homopolyester PGluxS is excluded. In fact, the maximum change observed for the onset temperature is a decrease in 10 °C whereas the maximum rate decomposition temperature slightly increases with copolymerization. The fact that opposite tendencies are observed for oTd and maxTd respectively, suggests the presence of small amounts of structural water associated to the Glux units in PBxGluxyS copolyesters. The exceptional behavior observed for PGluxS can be explained by assuming that it contains adsorbed water in much larger amounts than copolyesters, a conjecture that makes much sense given the 100 mole% content of this homopolyester in Glux units. The high heat resistance displayed by PBxGluxyS is a really remarkable fact regarding the potential of these copolyesters to be used in applications involving thermal processing.
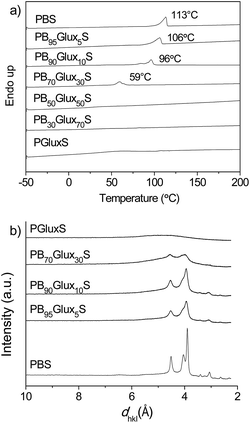
The glass transition and melting temperatures of PBxGluxyS copolyesters and homopolyesters were measured by DSC. Observation of the slope changes were clearly seen for the whole series on traces recorded from samples quenched from the melt that were exempted of crystallinity (see Fig. SI-4†). The Tg observed for copolyesters varied from −28 to 80 °C with values steadily increasing for increasing contents in Glux units (Table 2). This range of values is fully consistent with the Tg values displayed by the parent homopolyesters (−37 °C and 103 °C for PBS and PGluxS, respectively). Such strong enhancing effect is just simply the consequence of the increasing in chain stiffness that is produced when the flexible butylene segment is replaced by the rigid bicyclic Glux structure.
The influence of copolymerization on the melting/crystallization behavior was brought into evidence by DSC. As it is shown in Fig. 4a, the DSC heating traces of copolyesters with contents in Glux units of 30 mole% as maximum displayed an endothermic peak characteristic of melting and revealed therefore that they are semicrystalline. Both Tm and ΔHm decreased as the presence of Glux units increased. Copolyesters with Glux contents above 30 mole% as well as the PGluxS homopolyester produced plain traces without any vestige of crystallinity. This tendency is a consequence of the depressing effect on chain regularity that is produced when butylene units are replaced by Glux units.
The X-ray diffraction analysis corroborated the DSC results by showing discrete scattering diffraction for PBS and PBxGluxyS copolyesters containing up to 30% of Glux units with a peak sharpness and intensity decreasing with the increasing B/Glux ratio (Fig. 4b). Moreover the reflections observed for the semicrystalline copolyesters were coincident in both spacing and intensity with those characteristic of PBS,23 which is indicative that the crystal structure of the homopolyester is retained after copolymerization.
The trend to crystallize is a relevant property of semicrystalline polyesters that has to be considered when they are intended to be used as thermoplastics. As it can be seen in Table 2 only PBxGluxyS copolyesters containing 10 mole% of Glux as maximum are able to crystallize from the melt (Fig. SI-5†). Although these results clearly indicated that crystallizability of PBS is strongly depressed by the insertion of Glux units in the polyester chain, and that such effect has been reported to invariably occur for other related copolyesters containing sugar units, a comparative crystallization kinetics study has been undertaken in this work to quantify the influence of Glux in this regard.
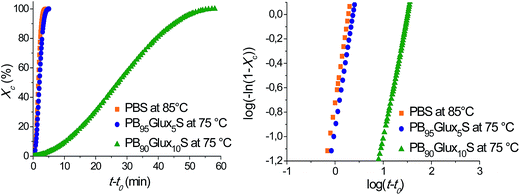
PBS, PB95Glux5S and PB90Glux10S were compared regarding their isothermal crystallization although a common temperature could not be set for the three compounds due to their large differences in crystallizability. The study also included the crystallization of each polymer at two different crystallization temperatures in order to estimate the influence of temperature on crystallization rate. The graphical representations of crystallization data as a function of time are depicted in Fig. 5 for the three compared polyesters. The kinetics was evaluated by the classical Avrami model,24,25 and the crystallization half-times afforded by this analysis are given in Table 2. It is clearly noticeable how t1/2 is strongly influenced by composition so it increased more than ten times for an increase in the Glux content of only 5 mole%. On the other hand, the observed inverse dependence of crystallization rate on temperature indicates that in both PBS and copolyesters, the crystallization process is controlled mainly by nucleating factors rather than by chain mobility.24
Stress–strain behavior
For a preliminary assessment of the mechanical behavior of PBxGluxyS, films of the copolyesters were subjected to stress–strain essays testing. Considering the strong influence that crystallinity has on mechanical properties, the samples used for tensile testing were previously checked by DSC. These measurements proved that melting parameters (Tm and ΔHm) of films were close to those recorded for the powdered samples with the initial PBS crystallinity decreasing about 20, 30 and 50% for 5, 10 and 30 mole% of Glux in the copolyester, respectively, to finally disappear for higher contents. The copolyesters were tested in parallel and compared with PBS. The mechanical parameters obtained in these essays are gathered in Table 2. Results showed that the copolyesters with amounts of Glux units till 30 mole%, which are those displaying crystallinity, undergo a reduction in Young modulus and tensile strength whereas those with higher contents of Glux exhibit a sustained increasing in these parameters. Although rather wandering values were found for the elongation to break, probably due to sample heterogeneities and divergences in molecular weight, it can be reasonably concluded that ductility is not significantly modified by copolymerization. The ambiguous mechanical behavior exhibited by PBxGluxyS copolyesters reflects the ambivalent effect of the insertion of Glux unit in the PBS chain as far as crystallinity and chain mobility in the amorphous phase is concerning. For low contents in Glux, crystallinity is the main factor determining the stress–strain response, and E and σmax decrease with copolymerization. Conversely, for high Glux contents crystallinity disappears and Tg becomes the only property affecting deformation. Consequently the mechanical performance is improved with copolymerization to the point that amorphous PBxGluxyS copolyesters arrive to be stiffer and stronger than PBS.Hydrolytic degradation and biodegradation
In order to evaluate the effect of Glux on the behavior of PBS regarding its degradability and biodegradability, several essays were carried out in parallel using PBS, PGluxS and PB70Glux30S copolyester. Samples were incubated in the appropriate aqueous buffer solution, with or without lipases added, and degradation evolution was followed by monitoring the changes taking place in weight and molecular weight of the residue. Firstly, the degradation at pH 2.0 was performed to evaluate the influence of Glux on the chemical hydrolysis of PBS and results coming out from these essays have been plotted in Fig. 6a and a'. According to what should be expected for aliphatic polyesters, a continuous decreasing in both sample weight and polymer molecular weight is observed for the three polyesters along incubation time with the noticeable remark that changes became more accentuated for Glux containing polyesters. In second place the degradation of the three polyesters incubated under approximately physiological conditions both with and without porcine pancreas lipases added, was examined, and results obtained therein are presented also in Fig. 6b and b'. As expected, degradation at pH 7.4 took place in much less extent than at pH 2.0 but the changes observed in both W and Mn continued being of the same sign as before for the three tested polyesters. Interestingly, degradation was notably enhanced when lipases were added to the incubation medium to the point that changes taking place in W and Mn were comparable to those observed at pH 2.0. To get insight into the hydrolytic mechanism, the residue left by the PB70Glux30S after incubation at pH 2.0 for 40 days was analyzed by 1H NMR, which revealed that the content in Glux of this sample had decreased about 10%, i.e. about one third of the Glux units were released upon degradation. This result is demonstrative that hydrolysis of PBS containing Glux units mostly happens by breaking those ester groups in which the carbohydrate units are directly implied. Such a difference is also observed when the homopolyesters PGluxS and PManxS are compared (see Fig. SI-6†). All these results lead to conclude that not only the chemical degradation but also the biodegradability of PBS, becomes enhanced by the insertion of Glux units in the polyester chain.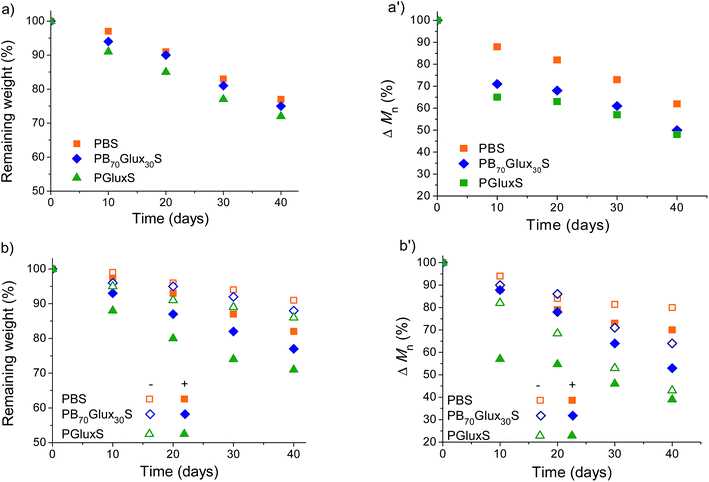 | ||
| Fig. 6 Degradation plots of PBS, PB70Glux30S, PGluxS at pH 2.0 (a and a') and pH 7.4 with (+) and without (−) porcine pancreas enzyme added (b and b'). | ||
Sugar-based bicyclic diols compared
The results achieved in this work have proven the ability of Glux-diol (2,4:3,5-di-O-methylene-D-glucitol) to be used as monomer for the preparation of PBS copolyesters with conveniently modified properties. A similar work with also satisfactory results but using Manx-diol (2,4:3,5-di-O-methylene-D-mannitol) has been recently published by us.21 It is of interest comparing these two bicyclic diols as comonomers of 1,4-butanediol for the preparation of PBS copolyesters.PBxGluxyS and PBxManxyS copolyesters synthesized by the same procedure have a random microstructure, their compositions are in general close to those used in their respective polymerization feeds, and their divergences regarding molecular weights are less than 15% (Fig. SI-7†). The two hydroxymethyl groups of Manx-diol are indistinguishable and equatorially oriented whereas in the asymmetrical Glux-diol, one CH2OH group is equatorial and the other one is axial. A recent study on the use of Glux-diol as comonomer in the solid state modification of PBT has shown that the reactivity of the axially oriented hydroxyl function in transesterification reactions was significantly hindered.26 However the slight differences in synthesis results attained for the two PBS copolyester series indicate that such hindering effect must not be significant in this case.
Although neither PBxGluxyS nor PBxManxyS copolyesters should be expected to be stereoregular due to the random distribution of the comonomers along their respective chains, the disorder will be less severe in the later due to the twofold symmetry of the Manx configuration. Accordingly PBxManxyS copolyesters show a greater ability to crystallize; they are crystalline over the whole range of compositions with crystallinity degrees oscillating between 50 and 65 mole%. As it is shown in Fig. 7a, Tm values in this series display a parabolic tendency with the minimum placed at comonomer compositions no far from 30 mole% and the maximum at 100 mole% (homopolyester PManxS). In contrast, only PBxGluxyS copolyesters containing 30 mole% of Glux units as maximum were found to be crystalline. Furthermore no sign of crystallinity was detected for PGluxS. Nevertheless practically identical Tm values are displayed by the two series over the interval in which PBxGluxyS are able to crystallize.
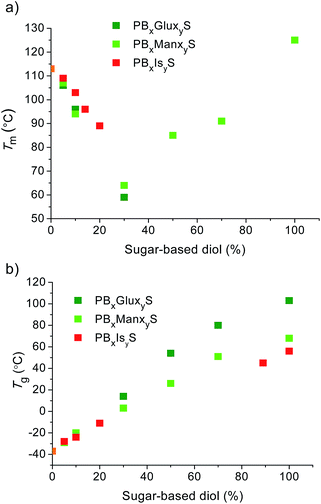 | ||
| Fig. 7 Compared melting (a) and glass transition (b) temperatures of PBS copolyesters made from Glux-diol, Manx-diol and isosorbide (Jacquel et al., 2015; Tan et al., 2011). PBS data in orange. | ||
A pronounced increase of Tg is perhaps the most interesting outcome of using bicyclic sugar-based compounds as comonomers in the synthesis of aliphatic polyesters. A close comparison of the Tg values displayed by PBxGluxyS and PBxManxyS series is graphically afforded in Fig. 7b. An almost linear trend is followed in both cases with slopes of ∼1.5 and ∼1.0 °C mole% sugar unit−1, respectively. The fact that higher Tg's are displayed by copolyesters containing Glux units is really amazing since they are less crystalline than their isocompositional Manx analogs. Apparently it is the more corrugated shape of the Glux structure which additionally contributes to hindering the mobility of the polyester chain and gives rise to an exceptionally increase in Tg.
Isosorbide (Is, 1,3:4,6-dianhydride-D-glucitol) is another glucose-derived bicyclic diol that has achieved in these last years wide recognition for the synthesis of bio-based semicrystalline copolyesters with high Tg.16,27 It will be worth therefore to compare Is with Glux and Manx regarding thermal properties. Unfortunately only a few papers dealing with PBS copolyesters containing isosorbide are found in the accessible literature,28,29 and data there afforded are incomplete or not fully suitable for a reliable comparison. Noordover et al.16b reported low molecular weight PIsS with Tg between ∼50 and ∼70 °C and Tan et al.29 described the a PB11Is89S copolyester with Mn 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and Tg of ∼45 °C. More recently, Jacquel et al.29 succeeded in preparing PBxIsyS copolyesters with astonishingly high molecular weights (45
000 and Tg of ∼45 °C. More recently, Jacquel et al.29 succeeded in preparing PBxIsyS copolyesters with astonishingly high molecular weights (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 < Mn < 55
000 < Mn < 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) although with compositions restricted to low contents in Is (less than 15 mole%). These copolyesters were reported to be semicrystalline with Tm decreasing with composition from 130 °C down to 89 °C and Tg increasing from −28 °C up to −11 °C. The Tm and Tg data available on PBxIsyS copolyesters have been also plotted in Fig. 7 for comparison with those of Glux and Manx. In spite of being scarce, data are enough to conclude that the effect of Is on PBS thermal properties is in line with that exerted by Manx and Glux.
000) although with compositions restricted to low contents in Is (less than 15 mole%). These copolyesters were reported to be semicrystalline with Tm decreasing with composition from 130 °C down to 89 °C and Tg increasing from −28 °C up to −11 °C. The Tm and Tg data available on PBxIsyS copolyesters have been also plotted in Fig. 7 for comparison with those of Glux and Manx. In spite of being scarce, data are enough to conclude that the effect of Is on PBS thermal properties is in line with that exerted by Manx and Glux.
Copolymerization of PBS with monocyclic and bicyclic sugar-based monomers has proven to be not only non-detrimental for its basic properties but favoring both chemical hydrolysis and biodegradation.21,30 The presence of the sugar moiety in the polyester chain does not deactivate the enzyme function but enhances its action due to increasing chain hydrophilicity. Both Manx and Glux have an enhancing effect on degradability upon aqueous incubation, either in absence or presence of lipases, but apparently Glux is significantly more efficient than Manx (see comparison in Fig. SI-8†). The higher enhancing effect displayed by Glux is most likely due to the strong depressing effect that this unit has on PBS crystallinity.
Conclusions
A series of PBS copolyesters (PBxGluxyS) containing bicyclic acetalized units derived from D-glucose (Glux) in addition to the homopolyester PGluxS were synthesized by melt polycondensation from mixtures of 1,4-butanediol, Glux-diol and dimethyl succinate. A complete incorporation of Glux-diol as well as satisfactory molecular weights were in general attained by careful selection of the reaction conditions. As it is usually observed for other sugar-based copolyesters, PBxGluxyS had a random microstructure. The presence of Glux in the polyester chain significantly modified the properties of PBS. Melting temperature and crystallinity were severely depressed so copolyesters containing more than 30 mole% of Glux including the homopolyester were amorphous. Oppositely, the glass transition temperature of PBS dramatically increased with the content in Glux units with a slope not paragoned by any other sugar-based unit described up to date. Mechanical properties of the sugar-based unit PBxGluxyS largely varying with composition with good results obtained for copolyesters with high contents in Glux. In line with the effect observed for other sugar-based copolyesters, PBxGluxyS display higher sensitivity to both hydrolytic degradation and biodegradation than PBS. All these results lead to finally conclude that Glux-diol is a highly appropriate bio-based comonomer to notably improve the properties of PBS as far as Tg and degradability are concerned. The exceptionally good accessibility of D-glucose as feedstock for Glux-diol is an additional merit of this compound if the modified PBS was intended to be used for industrial purposes.Acknowledgements
This work received financial support from MINECO of Spain with Grant MAT2012-38044-C03-03 and from AGAUR with grant 2009SGR1469. Thanks also to MECD of Spain for the Ph.D. grant awarded to Elena Zakharova.References
- A. Gandini, Green Chem., 2011, 13, 1061–1083 RSC.
- R. T. Mathers, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1–15 CrossRef CAS PubMed.
- E. S. Beach, C. Zheng and P. T. Anastas, Energy Environ. Sci., 2009, 2, 1038–1049 CAS.
- G.-Q. Chen and M. K. Patel, Chem. Rev., 2012, 112, 2082–2099 CrossRef CAS PubMed.
- A.-L. Marshall and P. J. Alaimo, Chem.–Eur. J., 2010, 16, 4970–4980 CrossRef CAS PubMed.
- K. Yao and C. Tang, Macromolecules, 2013, 46, 1689–1712 CrossRef CAS.
- A. Bhatia, R. Gupta, S. Bhattacharya and H. Choi, Korea-Aust. Rheol. J., 2007, 19, 125–131 Search PubMed.
- X. Kong, H. Qi and J. M. Curtis, J. Appl. Polym. Sci., 2014, 131, 40579 Search PubMed.
- (a) J. Li, X. Luo and X. Lin, Mater. Des., 2013, 46, 902–909 CrossRef CAS PubMed; (b) J. Li, X. Luo, X. Lin and Y. Zhou, Starch-Starke, 2013, 65, 831–839 CrossRef CAS PubMed.
- J. Xu and B.-H. Guo, Biotechnol. J., 2010, 5, 1149–1163 CAS.
- M. Snowdon, A. K. Mohanty and M. Misra, Macromol. Mater. Eng., 2015, 300, 118–126 CrossRef CAS PubMed.
- J. Yang, Q. Hao, X. Liu, C. Ba and A. Cao, Biomacromolecules, 2004, 5, 209–218 CrossRef CAS PubMed.
- (a) F. Bachmann, J. Reimer, M. Ruppenstein and J. Thiem, Macromol. Rapid Commun., 1998, 19, 21–26 CrossRef CAS; (b) F. Bachmann, J. Reimer, M. Ruppenstein and J. Thiem, Macromol. Chem. Phys., 2001, 202, 3410–3419 CrossRef CAS.
- M. Bueno, J. A. Galbis, M. G. García-Martín, M. V. Paz, F. Zamora and S. Muñoz-Guerra, J. Polym. Sci., Part A: Polym. Chem., 1995, 33, 299–305 CrossRef CAS PubMed.
- R. Quintana, A. Martínez de Ilarduya, A. Alla and S. Muñoz-Guerra, High Perform. Polym., 2012, 24, 24–30 CrossRef CAS PubMed.
- (a) B. A. J. Noordover, A. Heise, P. Malanowksi, D. Senatore, M. Mak, L. Molhoek, R. Duchateau, C. E. Koning and R. A. T. M. Benthem, Prog. Org. Coat., 2009, 65, 187–196 CrossRef CAS PubMed; (b) B. A. J. Noordover, V. G. Staalduinen, R. Duchateau, C. E. Koning, R. A. T. M. Benthem, M. Mak, A. Heise, A. E. Frissen and J. Haveren, Biomacromolecules, 2006, 7, 3406–3416 CrossRef CAS PubMed.
- (a) C. Lavilla, A. Alla, A. Martínez de Ilarduya, E. Benito, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Polymer, 2012, 53, 3432–3445 CrossRef CAS PubMed; (b) C. Lavilla and S. Muñoz-Guerra, Green Chem., 2013, 15, 144–151 RSC.
- S. Muñoz-Guerra, C. Lavilla, C. Japu and A. Martínez de Ilarduya, Green Chem., 2014, 16, 1716–1739 RSC.
- (a) C. Japu, A. Alla, A. Martínez de Ilarduya, M. G. García-Martín, E. Benito, J. A. Galbis and S. Muñoz-Guerra, Polym. Chem., 2012, 3, 2092–2101 RSC; (b) C. Japu, A. Martínez de Ilarduya, A. Alla, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Polym. Chem., 2014, 5, 3190–3202 RSC.
- (a) C. Lavilla, A. Martínez de Ilarduya, A. Alla, M. G. García-Martín, J. A. Galbis and S. Muñoz-Guerra, Macromolecules, 2012, 45, 8257–8266 CrossRef CAS; (b) C. Lavilla, A. Martínez de Ilarduya, A. Alla and S. Muñoz-Guerra, Polym. Chem., 2013, 4, 282–289 RSC.
- C. Lavilla, A. Alla, A. Martínez de Ilarduya and S. Muñoz-Guerra, Biomacromolecules, 2013, 14, 781–793 CrossRef CAS PubMed.
- R. Marín and S. Muñoz-Guerra, J. Appl. Polym. Sci., 2009, 114, 3723–3736 CrossRef PubMed.
- K. J. Ihn, E. S. Yoo and S. S. Im, Macromolecules, 1995, 28, 2460–2464 CrossRef CAS.
- G. Bodor, Structural investigation of polymers, Ellis Hordwood, Toronto, 1991, ch. 6, pp. 201–227 Search PubMed.
- J. A. Martins, W. Zhang and A. M. Brito, Rev. Sci. Instrum., 2005, 76, 105105 CrossRef PubMed.
- E. Gubbels, C. Lavilla, A. Martínez de Ilarduya, B. A. J. Noordover, C. E. Koning and S. Muñoz-Guerra, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 164–177 CrossRef CAS PubMed.
- R. Sablong, R. Duchateau, C. E. Koning, G. Wit, D. Es, R. Koelewijn and J. Haveren, Biomacromolecules, 2008, 9, 3090–3097 CrossRef CAS PubMed.
- L. Tan, Y. Chen, W. Zhou, J. Wei and S. Ye, J. Appl. Polym. Sci., 2011, 121, 2291–2300 CrossRef CAS PubMed.
- N. Jacquel, R. Saint-Loup, J.-P. Pascault, A. Rousseau and F. Fenouillot, Polymer, 2015, 59, 234–242 CrossRef CAS PubMed.
- E. Zakharova, C. Lavilla, A. Alla, A. Martínez de Ilarduya and S. Muñoz-Guerra, Eur. Polym. J., 2014, 61, 263–273 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. SI-1 1H NMR (top), 13C (bottom) spectra of PB90Glux10S copolyester with indication of peak assignments. Fig. SI-2 1H NMR (top), 13C (bottom) spectra of PGluxS homopolyester with indication of peak assignments. Fig. SI-3 derivative curves of PBS, PB90Glux10S and PB50Glux50S. Fig. SI-4 DSC traces of samples quenched from the melt for Tg observation. Fig. SI-5 DSC traces for PB95Glux5S. Fig. SI-6 degradation curves representing the decay in molecular weight against incubation time for PGluxS, PManxS at pH 7.4. Fig. SI-7 compared weight-average molecular weight of PBS copolyesters made from Glux-diol and Manx-diol. Fig. SI-8 degradation curves representing the decay in molecular weight against incubation time for isocompositional PBS copolyesters containing Glux and Manx units at pH 7.4. See DOI: 10.1039/c5ra03844h |
| This journal is © The Royal Society of Chemistry 2015 |