Synthesis of highly visible light active TiO2-2-naphthol surface complex and its application in photocatalytic chromium(VI) reduction
Peramaiah Karthika,
Ramalingam Vinotha,
Sundaram Ganesh Babua,
Meicheng Wenb,
Takashi Kamegawac,
Hiromi Yamashitab and
Bernaurdshaw Neppolian*a
aSRM Research Institute, SRM University, Kattankulathur, Chennai-603203, Tamil Nadu, India. E-mail: neppolian.b@res.srmuniv.ac.in; Fax: +91-44-2745-6702; Tel: +91-44-2741-7916
bDivision of Materials and Manufacturing Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
cNanoscience and Nanotechnology Research Center, Osaka Prefecture University, 1-2 Gakuencho, Nakaku, Sakai, Osaka 599-8570, Japan
First published on 24th April 2015
Abstract
Photocatalysis is an effective approach for the removal of heavy metal ions present in the aquatic bodies. In this report, TiO2 nanoparticles were successfully functionalized with 2-naphthol (2-NAP) using simple and scalable condensation reaction. The prepared photocatalyst was demonstrated as superior visible light photocatalyst for the effective reduction of Cr(VI). The 2-NAP functionalized TiO2 displayed a remarkable enhancement in the photocatalytic reduction of Cr(VI) under visible light irradiation (λ > 400 nm). The maximum Cr(VI) reduction of about 100% (7 fold higher activity than bare TiO2) was achieved within 3 h. The discernible enhancement in the photocatalytic reduction of TiO2-2-NAP can be ascribed to improved optical absorption in visible region, high crystallinity of TiO2 and high surface area. In addition, the photogenerated electron transfer from 2-NAP to TiO2 (ligand to metal transfer) can significantly improved the photocatalytic performance than bare TiO2 counterparts. Therefore, the functionalization of metal oxides with organic ligands can open new directions to overcome the existing limitations in photocatalysis.
1. Introduction
The heavy metals present in the aquatic system create adverse effects to the environment due to its highly toxic nature. Among the existing heavy metals, chromium (Cr) is the most commonly used metal for a variety of applications such as alloys, steel manufacturing, chrome plating, leather tanning and wood processing industries etc.1,2 In general, Cr exists in two variable oxidation states which are Cr(III) and Cr(VI). Compared to two Cr ions with different oxidation states, Cr(VI) is tremendously toxic and carcinogenic. Nonetheless, Cr(VI) is the most common contaminant present in the wastewater system which are directly discharged from the industries to the environment. As reported by the World Health Organization (WHO), the maximum tolerable concentration of Cr(VI) in drinking water is 0.05 mg L−1.3 Thus, it is a paramount important to reduce the highly toxic Cr(VI) to less harmful Cr(III). The techniques available for the removal and reduction of Cr(VI) are adsorption, ion-exchange, reverse osmosis, chemical precipitation, reduction with sodium sulfide and reduction by using ferrous sulfate, etc.4–9 However, most of these experimental techniques need sophisticated instrumentation, large quantity of chemicals and also it can produce the secondary wastes during the removal process.In recent years, photocatalytic reduction of Cr(VI) using semiconductor photocatalysts has been considered as a simple and an alternative technique for the efficient reduction of Cr(VI) to Cr(III). TiO2 based materials are the most promising photocatalysts for oxidation of organic pollutants and the removal of heavy metal ions from the aquatic bodies. However, TiO2 cannot be activated under visible light because of its wide bandgap which lies in the UV light region. The modification of TiO2 with visible light active metals, metal oxides, polymers and organic dye molecules are attracted as methods for design of efficient photocatalysts in the effective reduction of Cr(VI) as well as degradation of organic pollutants.10–17 Apart from these available methods, functionalization of TiO2 with organic ligands is a simple, alternative and scalable process for the reduction reaction. The condensation reaction between aromatic hydroxyl group and TiO2 surface hydroxyl group leads to the formation of ligand to metal charge transfer (LMCT) complex, which further excites electron from the highest occupied molecular orbital (HOMO) of aromatic ligand to the conduction band of TiO2 under visible light irradiation.18–21
Several similar strategies have been employed to improve the photocatalytic ability of TiO2 photocatalyst. Zhang et al. synthesized phenolic resin functionalized TiO2 by using the grafting method for hydrogen production in the presence of visible light.22 Yamashita et al. reported that the hydroxynaphthalene functionalized TiO2 showed successful reduction of 4-nitrophenol to 4-aminophenol.18 Koliyat and his co-workers reported that the TiO2–carbon based hybrid ligand to metal charge transfer complex prepared by N2 pyrolysis at 800 °C exhibited much higher photocatalytic activity than the bare TiO2 towards hydrogen production.23 Some research groups prepared visible light responsive amino-functionalised TiO2 by solvothermal method for both hydrogen production and nitrophenol reduction.24,25 Fu et al. synthesized visible light active amine functionalized TiO2 photocatalyst for CO2 reduction.24 Chen and co-workers modified the TiO2 surface with 2,4-diisocynate by using a surface chemical modification process for degradation of 2,4-dichlorophenol under visible light illumination.26 Jiang et al. synthesized the TiO2–phenolic resol hybrid material photocatalyst by solvothermal method for the degradation of methyl orange dye.27
In the present study, first time we have synthesized visible light active 2-naphthol (2-NAP) functionalized TiO2 using simple condensation reaction at ambient conditions. The synthesized TiO2-2-NAP showed effective reduction of Cr(VI) upon visible light irradiation. Hence, the present research work was mainly focused on synthesis, characterization studies and photocatalytic reduction properties of TiO2-2-NAP photocatalysts.
2. Experimental details
2.1. Materials
The titanium tetraisopropoxide was purchased from spectrochem chemicals (India) and potassium dichromate (K2Cr2O7), 2-naphthol analytical grade was purchased from SRL chemicals, India. All other solvents and reagents (analytical grade) were purchased and used without any further purification.2.2. Synthesis of mesoporous TiO2
The mesoporous TiO2 was synthesized as reported earlier.28 In brief, 0.032 mol titanium isopropoxide and 0.016 mol glacial acetic acid were dissolved in 20 mL of absolute ethanol. The mixture was allowed to stirr for 1 h. After stirring, the resultant suspension was added dropwise to a 100 mL deionised water under sonication. Subsequently, the whole colloidal mixture was sonicated for 3 h by low frequency ultrasonicator (3 s on, 1 s off, pulse mode, amplitude 35%) using a 13 mm diameter high intensity probe (Sonics and Materials UC 750, 20 KHz). The powder was collected by centrifugation, washed several times with deionised water and dried at 100 °C. The synthesized powder was calcined in air at 400 °C for 1 h.2.3. Synthesis of TiO2-2-NAP complex
The TiO2-2-NAP surface complex was synthesized using simple condensation reaction. 500 mg of prepared TiO2 was suspended in 10 mL of acetone. To the above suspension, required amount (different wt%) of 2-NAP was added drop by drop under continuous stirring. The resulting suspension was allowed to stirr for 1 h at room temperature. Then, the color of the reaction mixture was changed to light yellow, which indicating the formation of TiO2-2-NAP complex. Finally, the product was collected by centrifugation and washed several times with acetone to remove residual organic moiety and dried at 40 °C.2.4. Characterization studies
The synthesized TiO2-2-NAP complex was characterized using various spectroscopic techniques. X-ray diffraction (XRD) patterns were recorded from PANalticalX'pert powder diffractometer using Cu Kα radiation. Diffuse Reflectance Spectra (DRS) were monitored by UV-2600 spectrophotometer (Shimadzu) in DRS mode. The morphological studies of the prepared photocatalysts were carried out by using Field Emission-Scanning Electron Microscopy (FEI Quanta FEG 200 HR-SEM). The surface area was obtained by using a BEL-SORP max (BEL Japan, Inc.). Fourier Transform Infrared (FT-IR) spectra were obtained using Perkin-Elmer-USA.2.5. Photocatalytic reduction studies
The photocatalyst powder (100 mg) was suspended in 100 mL of 30 ppm standard Cr(VI) solution, which is prepared from potassium dichromate crystals. Before the addition of photocatalyst, the pH of the solution was adjusted to 7 by the addition of 0.1 M NaOH. The resultant mixture was allowed to stirr for 30 min at dark to reach the adsorption–desorption equilibrium. After stirring, the suspension was irradiated with 150 W visible lights (λ > 400 nm) with constant stirring at room temperature. The visible lamp was placed into a quartz double-wall water circulation jacket to avoid overheating and the whole setup was immersed into the chromium solution taken in a 250 mL beaker. However, The intensity of light was measured using Lux meter and the intensity was 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Lux. During the photocatalytic reaction, at a fixed interval of time, 3 mL sample was collected, centrifuged and analyzed by UV-visible spectrophotometer (Specord 200 plus, Analytik Jena, Germany) to quantify the concentration of Cr(VI). Presence of Cr ions before and after the photocatalytic reaction was estimated using atomic absorption spectroscopy (AAS) which is recorded using Agilent Verne – 210, USA.
000 Lux. During the photocatalytic reaction, at a fixed interval of time, 3 mL sample was collected, centrifuged and analyzed by UV-visible spectrophotometer (Specord 200 plus, Analytik Jena, Germany) to quantify the concentration of Cr(VI). Presence of Cr ions before and after the photocatalytic reaction was estimated using atomic absorption spectroscopy (AAS) which is recorded using Agilent Verne – 210, USA.
3. Results and discussion
3.1. XRD Pattern
The XRD patterns of P25 (Degussa TiO2), TiO2 and TiO2-2-NAP are depicted in Fig. 1. The commercial P25 shows the characteristic diffraction peaks at 25 and 28°, indicating that the P25 is composed of both anatase and rutile phase structures.29 The mesoporous TiO2 prepared using ultrasound exhibits the characteristic diffraction peaks at 25.1, 37.8, 47.9, 53.9, 55.3, 62.6, 68.8, 70.2, 75.2 and 82.8° correspond to (101), (004), (200), (105), (211), (204), (116), (220), (215) and (224) planes, respectively. These observed XRD peaks clearly indicate the formation of well crystalline anatase phase TiO2 structure (JCPDS card no. 89-4921).30 However, no additional peaks or shift in the existing peaks of anatase phase of TiO2 was observed with the 2-NAP functionalized TiO2 photocatalyst, which indicates that the functionalization of TiO2 with 2-NAP did not affect or induce any new crystalline phase in the TiO2 structure.313.2. SEM and EDX
The morphology of the prepared TiO2 and TiO2-2-NAP photocatalysts were investigated by SEM analysis. The SEM micrographs are displayed in Fig. 2a & b. It can be clearly seen that the irregular shaped particles are obtained for both TiO2 and TiO2-2-NAP. However, the 2-NAP functionalized TiO2 has no specific influence on the morphological change of TiO2. Fig. 2c & d gives the EDX profile of TiO2 and TiO2-2-NAP photocatalysts. The prepared TiO2-2-NAP is composed of Ti, O and C respectively. Therefore, the existence of C in the present result clearly confirms the functionalization of 2-NAP with TiO2. | ||
| Fig. 2 SEM image of (a) TiO2, (b) TiO2-2-NAP and EDX spectra of (c) TiO2 [inset: elemental weight percentage table] (d) TiO2-2-NAP [inset: elemental weight percentage table]. | ||
3.3. UV-vis-DRS spectra
Fig. 3 displays the UV-vis diffuse reflectance spectra of the P25, TiO2 and 2-NAP functionalized TiO2. The absorption edge for P25, TiO2 and TiO2-2-NAP are observed at 379, 389 and 407 nm, respectively. Compared to P25 and TiO2, the red-shift in the absorption edge of TiO2-2-NAP is obtained due to the formation of TiO2-2-NAP LMCT complex. As shown in Fig. 3, the band gap energy was calculated from the corresponding absorption edges of P25, TiO2 and TiO2-2-NAP. The bandgap energies of P25, TiO2 and TiO2-2-NAP was estimated to be 3.2, 3.1 and 3.0 eV, respectively. The slight red-shift in the bandgap is mainly due to the formation of the LMCT complex between surface hydroxyl group of TiO2 and 2-NAP. Moreover, TiO2-2-NAP has a clear optical response in the visible light area ranging from 400 nm to 550 nm, which further demonstrates that TiO2-2-NAP is more capable to drive the photocatalytic reaction under visible light irradiation compared to that of bare P25 and TiO2 alone.3.4. BET-surface area analysis
Fig. 4 gives the N2 adsorption–desorption isotherms of P25, TiO2 and TiO2-2-NAP photocatalyst. The surface areas of photocatalysts are given in Table 1. As can be seen from Table 1, the surface area of P25, TiO2 and TiO2-2-NAP was observed to be 46.1, 133 and 130 m2 g−1, respectively. This clearly reveals that the photocatalyst prepared in the presence of ultrasound have more surface area, whereas the P25 has the less surface area. Similar result has been reported by Neppolian et al.32 However, there is no significant change in surface area was observed with the 2-NAP functionalized TiO2. The pore diameters of meso-TiO2 and TiO2-2-NAP were 8.79 and 8.77 nm. Larger surface area and smaller pore diameter can make this photocatalyst more efficient for the effective reduction of Cr(VI).| Entry | Name of samples | SBET (m2 g−1) | Vp (cm3 g−1) | Pore size (nm) |
|---|---|---|---|---|
| 1 | TiO2-2-NAP | 130 | 0.286 | 8.79 |
| 2 | TiO2 | 133 | 0.291 | 8.77 |
| 3 | P25 | 46.1 | 0.357 | 31.0 |
3.5. FT-IR
To further confirm the LMCT surface complex formation between 2-NAP and TiO2, FTIR spectra were recorded in attenuated total reflectance (ATR) mode. The FTIR spectra of free 2-NAP and TiO2-2-NAP are illustrated in Fig. 5a & b. As shown in Fig. 5a, the peaks centered at 1632, 1601, 1583, 1512, 1466 and 1408 cm−1 are attributed to the stretching vibrations of the aromatic ring (C–C) and (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) bonds. Similarly, the observed bands at 1118, 1138, 1172, 1217, 1242, 1277, 1323, 1363 and 1379 cm−1 correspond to the stretching or bending vibration of the phenolic C–OH group. In the case of TiO2-2-NAP, the intensity of phenolic vibration peaks at 1138, 1172, 1217, 1242, 1277, 1323, 1363 and 1379 cm−1 are lower than pure 2-NAP (Fig. 5b).18,33,34 This observation clearly revealed the formation of surface complexes between the hydroxyl groups of both TiO2 and 2-NAP through simple dehydration reaction.
C) bonds. Similarly, the observed bands at 1118, 1138, 1172, 1217, 1242, 1277, 1323, 1363 and 1379 cm−1 correspond to the stretching or bending vibration of the phenolic C–OH group. In the case of TiO2-2-NAP, the intensity of phenolic vibration peaks at 1138, 1172, 1217, 1242, 1277, 1323, 1363 and 1379 cm−1 are lower than pure 2-NAP (Fig. 5b).18,33,34 This observation clearly revealed the formation of surface complexes between the hydroxyl groups of both TiO2 and 2-NAP through simple dehydration reaction.
3.6. Photocatalytic reduction of Cr(VI)
The photocatalytic reduction of Cr(VI) was carried out to evaluate the photoreduction ability of 2-NAP functionalized TiO2 photocatalyst. Fig. 6a & b shows the decrease in the absorption spectrum of Cr(VI) in the presence of bare TiO2 and TiO2-2-NAP upon visible light irradiation (λ > 400 nm). As shown in Fig. 6a, the bare meso TiO2 exhibits lower photocatalytic reduction of Cr(VI), whereas the 2-NAP functionalized TiO2 photocatalyst shows complete reduction (100%) of Cr(VI) within 3 h of visible light irradiation [Fig. 6b]. Recently, similar reduction efficiency was observed by Mondal et al. using SnS2 photocatalyst.35 For a comparison, the photocatalytic reduction reaction was carried out with P-25 and its exhibited only 12% reduction under similar conditions. Hence, the substantial enhancement in the photoreduction behavior of TiO2-2-NAP, can be justified that the 2-NAP induces the charge transfer (CT) between the HOMO of 2-NAP to conduction band of TiO2. | ||
| Fig. 6 UV-vis absorption spectra of Cr(VI) reduction using (a) meso-TiO2 and (b) TiO2-2-NAP (1 wt%). | ||
As shown in Fig. 7, our preliminary tests demonstrated that no reduction reaction is observed in the absence of either light or photocatalyst alone, suggesting that the reaction was mainly driven by photocatalysis, whereas around 20% of adsorption is noted in dark condition after 3 h. In order to study the effect of 2-NAP in Cr(VI) reduction, different weight percents (0.5, 1, 1.5 and 2.0 wt%) of 2-NAP was loaded and their photocatalytic activity was evaluated under identical experimental conditions. As shown in Fig. 7, the photocatalytic reduction efficiency of 0.5, 1, 1.5 and 2.0 wt% of 2-NAP loaded TiO2 are 67, 100, 85, and 76%, respectively. It can be clearly seen that 1 wt% 2-NAP loaded photocatalyst exhibits remarkable enhancement in the Cr(VI) reduction than the other composites. However, the higher concentration of 2-NAP loaded TiO2 decreases its photocatalytic activity. This might be due steric hindrance and hence it may suppress the electron transfer from the aromatic ring of 2-NAP to TiO2. However, the excellent photocatalytic activity of TiO2-2-NAP composites can be ascribed due to the direct electron ejection from the HOMO of 2-NAP to the conduction band of TiO2. Moreover, it also an evidence for the LMCT complex formation between the TiO2 and 2-NAP as proven by FT-IR spectrum.
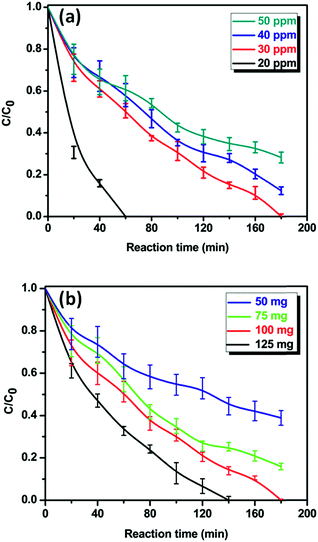
3.7. Effect of initial concentration of Cr(VI) and different amount of catalyst loading
The effect of initial concentration of Cr(VI) on photocatalytic reduction was carried out by varying the initial concentration of Cr(VI) from 20 to 50 ppm and the catalyst loading was kept constant. The change in Cr(VI) concentration with respect to the irradiation time is shown in Fig. 8a. As can be seen from Fig. 8a, the photocatalytic reduction rate of Cr(VI) is gradually decreasing from 100 to 64% by increasing the initial concentration from 20 to 50 ppm. The rate constant values for the photoreduction of Cr(VI) with different concentrations (20–50 ppm) using TiO2-2-NAP photocatalyst were calculated from Fig. 8a and the results are summarized in Table 2. As can be seen from Table 2 that the rate constant decreases with increase in the initial concentration of Cr(VI). This result clearly reveals that the photocatalytic reduction of Cr(VI) using TiO2-2-NAP photocatalyst depends on the initial concentration of Cr(VI) and follows pseudo first order reaction kinetics. However, 20 ppm concentration is very high concentration in comparison with the naturally available chromium or present in different industrial effluents. This result clearly emphasizing that the initial concentration of Cr(VI) plays a significant role in the photoreduction process. Since the reaction time and the amount of catalyst are constant, the photo-generated electrons on the surface of the catalyst is also constant. Therefore, the relative number of electrons reacting with Cr(VI) decreases with increasing concentration of the chromium. Moreover, the reduced Cr ions can occupy the catalytic active sites of photocatalysts, which results in lower photocatalytic activity.36,37 The another possible reason is that the increase in the initial concentration of Cr(VI) can easily prevent the penetration of light to the surface of photocatalysts.38| Entry | Initial concentration of Cr(VI) (ppm) | k′ (min−1) | R2 |
|---|---|---|---|
| 1 | 20 | 0.046 | 0.9470 |
| 2 | 30 | 0.013 | 0.9860 |
| 3 | 40 | 0.010 | 0.9665 |
| 4 | 50 | 0.007 | 0.9732 |
To investigate the effect of photocatalyst loading on the photocatalytic reduction of Cr(VI), the catalyst loading amount was varied from 50 to 125 mg without changing the initial concentration of Cr(VI). It is clearly seen from Fig. 8b, the photocatalytic reduction rate of Cr(VI) is gradually increased from 56, 77 and 100% with increase in catalyst dosage (50 to 125 mg). This phenomenon can be clearly explained that the increase in catalyst loading can absorb more incident light to enhance the charge transfer between aromatic rings of 2-NAP and the conduction band of TiO2, which further facilitate the high photocatalytic activity.
3.8. Atomic absorption spectroscopy
In order to confirm the reduction of Cr(VI) to Cr(III) by this photocatalytic route, AAS measurements were carried out before and after the photocatalytic reaction (Fig. 9). For this purpose, 20 ppm Cr(VI) solution at neutral pH was irradiated with visible light in the presence of TiO2-2-NAP photocatalyst. The initial and final solution was tested using AAS to estimate the Cr concentration and was found to be 19.72 and 18.68 ppm, respectively. This result confirmed the presence of Cr in the final solution, after the visible light driven photoreaction. However, the formation of Cr(III) ion was authenticated by adjusting the pH of the initial and final reaction medium to 10.5 using 1 M NaOH solution. After adjusting the pH to 10.5, the Cr(III) ion present in the final solution precipitated as Cr(OH)3 which was removed by filtration (using 0.22 micron filter paper) before AAS analysis. The AAS results suggested the presence of 19.91 ppm of Cr ions in the initial solution at pH = 10.5 whereas the final solution showed only 0.65 ppm. This substantiated the reduction of Cr(VI) ions by this photocatalytic system in presence of TiO2-2-NAP and also strongly proved the presence of Cr(III) ions.3.9. Plausible photocatalytic reaction mechanism
On the basis of the above results and discussion, a plausible mechanism of visible light induced LMCT is proposed to explain the photocatalytic activity of the TiO2-2-NAP surface complex as illustrated in the Fig. 10. Generally, visible light activation of wide bandgap semiconductors (like TiO2, ZnO, etc.) can be achieved by using surface adsorbates (either dyes or complexes) as sensitizers other than coupling with narrow bandgap semiconductors. The photochemical process of dye sensitization is usually initiated by HOMO to LUMO photoexcitation of dye molecules, followed by electron transfer from the LUMO level of the excited dye to TiO2 conduction band (CB). On the other hand, the visible light mediated charge transfer occurs from the HOMO of adsorbates to the CB of TiO2, which is referred to as the ligand-to-metal charge transfer (LMCT) process.39 Hence, unlike the case of dye sensitization where the sensitizer itself should absorb the visible light, a variety of organic or inorganic compounds (that do not absorb visible light) can be a potential LMCT sensitizer. In the present study, when visible light strikes on the TiO2-2-NAP complex, the electrons present in the HOMO of 2-NAP are directly excited to the conduction band of TiO2. Hence, the population of electron increases in the conduction band of TiO2, which is subsequently induce the Cr(VI) to Cr(III) reduction. Recently, Tachan et al. proposed similar LMCT mechanism for visible light active catechol functionalized TiO2 photocatalyst.40 Moreover, the similar LMCT mechanism has also been reported by other researchers for the different photocatalytic applications.41–43 | ||
| Fig. 10 A possible mechanism of photocatalytic Cr(VI) reduction under visible light irradiation using TiO2-2-NAP. | ||
4. Conclusion
A visible light sensitive TiO2-2-NAP surface complex was successfully synthesized via simple condensation reaction method. The synthesized 2-NAP functionalized TiO2 exhibited 100% photocatalytic activity for the Cr(VI) reduction. The higher photocatalytic performance was mainly attributed due to the functionalization of 2-NAP on TiO2. The optimal amount of 2-NAP functionalization was determined to be 1 wt%. A plausible mechanism of the photocatalytic Cr(VI) reduction process was proposed. This study opens a new possibility in the investigation of TiO2-2-NAP surface complex and promotes their practical applications in environmental issues.Acknowledgements
We gratefully acknowledge SERB-DST (DST no. SR/FT/CS-127/2011), New Delhi, India for the financial support.References and Notes
- L. B. Khalil, W. E. Mourad and M. W. Rophae, Appl. Catal., B, 1998, 17, 267 CrossRef CAS.
- N. Shevchenko, V. Zaitsev and A. Walcarius, Environ. Sci. Technol., 2008, 42, 6922 CrossRef CAS.
- WHO, Geneva 3/e, 2004, 334.
- M. Dakiky, M. Khamis, A. Manassra and M. Mereb, Adv. Environ. Res., 2002, 6, 533 CrossRef CAS.
- M. Emine, N. Yasar and D. Murat, J. Hazard. Mater., 2006, 138, 142 CrossRef PubMed.
- S. A. Cavaco, S. Fernandes, M. M. Quina and L. M. Ferreira, J. Hazard. Mater., 2007, 144, 634 CrossRef CAS PubMed.
- S. A. M. Rad, S. A. Mirbagheri and T. Mohammadi, Using reverse osmosis membrane for chromium removal from aqueous solution, World Academy of Science, Engineering and Technology, 2009, vol. 3, p. 28 Search PubMed.
- L. L. Skovbjerg, S. L. S. Stipp, S. Utsunomiy and R. C. Ewing, Geochim. Cosmochim. Acta, 2006, 70, 3582 CrossRef CAS PubMed.
- R. D. Ludwig, C. Su, T. R. Lee, R. T. Wilkin, S. D. Acree, R. R. Rose and A. Keeley, Environ. Sci. Technol., 2007, 41, 5299 CrossRef CAS.
- D. Zhang, X. Li, H. Tan, G. Zhang, Z. Zhao, H. Shi, L. Zhang, W. Yub and Z. Sun, RSC Adv., 2014, 4, 44322 RSC.
- S. G. Babu, R. Vinoth, B. Neppolian, D. D. Dionysiou and M. Ashokkumar, J. Hazard. Mater., 2015, 291, 83 CrossRef CAS PubMed.
- S. Kim, S. J. Hwang and W. Choi, J. Phys. Chem. B, 2005, 109, 24260 CrossRef CAS PubMed.
- J. Yu, G. Dai and B. Huang, J. Phys. Chem. C, 2009, 113, 16394 CAS.
- H. Park and W. Choi, Langmuir, 2006, 22, 2906 CrossRef CAS PubMed.
- J. Zhang, J. H. Bang, C. Tang and P. V. Kamat, ACS Nano, 2010, 4, 387 CrossRef CAS PubMed.
- H. Yamashita, M. Harada, J. Misaka, M. Takeuchi, B. Neppolian and M. Anpo, Catal. Today, 2003, 84, 191 CrossRef CAS.
- B. Neppolian, H. Jung and H. Choi, J. Adv. Oxid. Technol., 2007, 10, 2 Search PubMed.
- T. Kamegawa, H. Seto, S. Matsuura and H. Yamashita, ACS Appl. Mater. Interfaces, 2012, 4, 6635 CAS.
- J. Moser, S. Punchihewa, P. P. Infelta and M. Gratzel, Langmuir, 1991, 7, 3012 CrossRef CAS.
- P. Persson, R. Bergstrom and S. Lunell, J. Phys. Chem. B, 2000, 104, 10348 CrossRef CAS.
- I. A. Jankovic, Z. V. Saponjic, M. I. Comor and J. M. Nedeljkovic, J. Phys. Chem. C, 2009, 113, 12645 CAS.
- G. Zhang and W. Choi, Chem. Commun., 2012, 48, 10621 RSC.
- S. K. Parayil, H. S. Kibombo and R. T. Koodali, Catal. Today, 2013, 199, 8 CrossRef CAS PubMed.
- Y. Fu, D. Sun, Y. Chen, R. Huang, Z. Ding, X. Fu and Z. Li, Angew. Chem., Int. Ed., 2012, 51, 3364 CrossRef CAS PubMed.
- T. Toyao, M. Saito, Y. Horiuchi, K. Mochizuki, M. Iwata, H. Higashimura and M. Matsuoka, Catal. Sci. Technol., 2013, 3, 2092 CAS.
- F. Chen, W. Zou, W. Qu and J. Zhang, Catal. Commun., 2009, 10, 1510 CrossRef CAS PubMed.
- Y. Jiang, L. Meng, X. Mu, X. Li, H. Wang, X. Chen, X. Wang, W. Wang, F. Wu and X. Wang, J. Mater. Chem., 2012, 22, 23642 RSC.
- J. C. Yu, L. Zhanga and J. Yua, New J. Chem., 2002, 26, 416 RSC.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2010, 4, 380 CrossRef CAS PubMed.
- B. Neppolian, Q. Wang, H. Jung and H. Choi, Ultrason. Sonochem., 2008, 15, 649 CAS.
- D. Jianga, Y. Xua, D. Wua and Y. Suna, J. Solid State Chem., 2008, 181, 593 CrossRef PubMed.
- B. Neppolian, E. Celik, M. Anpo and H. Choi, Catal. Lett., 2008, 125, 183 CrossRef CAS.
- T. D. Savi, Z. V. Saponjic, M. I. Comor, J. M. Nedeljkovi, M. D. D. Canin, M. G. N. Dusan, Z. Veljkovi, S. D. Zari and I. A. Jankovi, Nanoscale, 2013, 5, 7601 RSC.
- T. D. Savi, I. A. Jankovi, Z. V. Saponji, M. I. Comor, M. G. N. Dusan, Z. Veljkovi, S. D. Zari and J. M. Nedeljkov, Nanoscale, 2012, 4, 1612 RSC.
- C. Mondal, M. Ganguly, J. Pal, A. Roy, J. Jana and T. Pal, Langmuir, 2014, 30, 4157 CrossRef CAS PubMed.
- A. Akyol, H. C. Yatmaz and M. Bayramoglu, Appl. Catal., B, 2004, 54, 19 CrossRef CAS PubMed.
- M. S. Siboni, M. Farrokhi, R. D. C. Soltani, A. Khataee and S. Tajassosi, Ind. Eng. Chem. Res., 2014, 53, 1079 CrossRef.
- M. Qamara, M. A. Gondala and Z. H. Yamania, J. Mol. Catal. A: Chem., 2011, 341, 83 CrossRef PubMed.
- V. H. Houlding and M. Gratzel, J. Am. Chem. Soc., 1983, 105, 5695 CrossRef CAS.
- Z. Tachan, I. Hod and A. Zaban, Adv. Energy Mater., 2014, 4, 1301249 Search PubMed.
- X. Li, C. Chen and J. Zhao, Langmuir, 2001, 17, 4118 CrossRef CAS.
- G. Kim and W. Choi, Appl. Catal., B, 2010, 100, 77 CrossRef CAS PubMed.
- G. Zhang, G. Kim and W. Choi, Energy Environ. Sci., 2014, 7, 954 CAS.
| This journal is © The Royal Society of Chemistry 2015 |