One-pot preparation of nanocrystalline Ag–WO3 catalyst for the selective oxidation of styrene†
Shilpi
Ghosh
a,
Shankha S.
Acharyya
a,
Malika
Kumar
b and
Rajaram
Bal
*a
aCatalytic Conversion & Processes Division, CSIR-Indian Institute of Petroleum, Dehradun-248005, India. E-mail: raja@iip.res.in
bM. Tech. Chemical Synthesis & Process Technologies (CSPT), Department of Chemistry, University of Delhi, New Delhi, India
First published on 16th April 2015
Abstract
Cationic surfactant cetyltrimethylammonium bromide-mediated water-based preparation of nanocrystalline Ag–WO3 catalyst has been reported in a one-pot preparation method. This catalyst has been characterized by XRD, XPS, SEM, TEM, STEM-mapping, FTIR, Raman, TGA, and ICP-AES. SEM images displayed the formation of an aloevera-like structure. TEM-images revealed the formation of ultrasmall Ag (with average size 5 nm), anchored on monoclinic WO3 rods (with diameters in the range between 80 and 120 nm). It was found that the catalyst can effectively oxidize styrene with H2O2 as the oxidant. The effects of different reaction parameters have been studied in detail. A styrene conversion of 75% with a styrene-oxide selectivity of 55% and a styrene conversion of >99% with a benzaldehyde selectivity of 88% were accomplished over this catalyst, varying different reaction conditions. The catalyst did not show any leaching for up to five reuses, showing the true heterogeneity of the catalyst. However, significant H2O2 decomposition occurs on the catalyst necessitating its usage in four-fold excess.
One-dimensional (1D) nanostructures (such as wires, rods, tubes, and belts) have been the focus of extensive research in recent years due to their potential applications in fabricating nanoscale electronic, optoelectronic, sensing devices and catalysis. More recently, many efforts have been devoted to the controlled synthesis and assembly of metal nanowires owing to their promising use as interconnects or active components in fabricating nano-devices and their important roles in probing a variety of physical phenomena.1–3 Additionally, the surface of 1D nanomaterials is inherently rich in coordinatively unsaturated sites that can play an active role in catalytic reactions.4 Thus, 1D nanomaterials are drawing growing attention for the specific physical properties that they display compared to their bulk counterparts. Solution-phase techniques (wet chemistry) have been shown to be a very advantageous and viable approach for the preparation of metal-oxide nanomaterials.4 However, these methods typically require the use of templates or other additives to direct the growth of the material towards a specific morphology. Cetyltrimethyl ammonium bromide (CTAB) is a cationic surfactant that plays an important role in controlling the formation of micro and nano architectures under the template effect (soft template). The growth of the certain architecture is associated with the selective interaction of the organic surfactants on certain crystallographic facets to stimulate the crystal growth.5,6
Herein, we report a one-pot, CTAB-mediated, facile aqueous approach to prepare a forest of oriented silver–tungsten aloevera type material, that consists of numerous nanorods (1D) at room temperature. The morphology of the diameter and length of the nanorods in the system can be tuned by varying the experimental parameters. To the best of our knowledge, there is no literature present that reported the synthesis of this type of 1D composite nanomaterial previously.
Direct functionalization of hydrocarbons by catalytic oxidation of C–H bonds to form oxygenated products under mild conditions is a major challenge, since this path serve as the key to the formation of value-added oxygenated chemicals and pharmaceuticals.7,8 Among many oxidation reactions, catalytic oxidation of styrene is one of the most notable examples since the reaction gives valuable oxygenated compounds, which can serve as precursors for many chemical products like styrene oxide, acetophenone and benzaldehyde, and even benzoic acid.9 Among these oxygenates styrene oxide and benzaldehyde are the most important oxygenates. Styrene oxide, which is an important intermediate for large variety of fine chemicals such as perfumes and drugs etc. Traditionally, oxidation of styrene is carried out by using stoichiometric amounts of organic peracids as oxidant.10 On the other hand, benzaldehyde (almond aroma) is the second most used flavouring agent.11 In 2009, it was estimated that 90 kilotons of benzaldehyde were synthesized through industrial processes hampered by high temperatures and pressures that nevertheless resulted in lackluster yields.11 One industrial pathway to benzaldehyde is the hydrolysis of benzalchloride synthesis which generates large quantities of HCl as a by-product.12 The alternative, and more popular industry method of air-oxidation of toluene, results in low conversions of starting materials and produces benzaldehyde only as a by-product in the production of the less valuable benzoic acid.12 But production of a specific oxygenate selectively, with high yield is a great challenge to the researchers; so, many researchers came forward to sort out the problem using various oxidants like TBHP,13–15 molecular oxygen,11,16,17 and a mixture of TBHP and molecular oxygen.18 In contrast to the said oxidants, use of H2O2-based catalytic epoxidation is of great advantage to the environment and industry because (i) it generates H2O as a sole by-product, (ii) it has a high content of active oxygen species (47%), and (iii) H2O2 is rather inexpensive compared with organic peroxides and peracids19 and several researchers reported styrene-oxidation with H2O2 as oxidant. Laha et al. demonstrated that, the yield of styrene oxide from styrene can be increased to ∼52% if urea is mixed with H2O2 as oxidant, using TS-1 catalyst.20 Rajabi et al. reported ∼95% yield of benzaldehyde from styrene using supported Fe-nanoparticles and H2O2 as oxidant.21 Duarte et al. reported ∼58% yield of benzaldehyde using tetrabutylammonium salt of [XW11M(H2O)O39](n−m)−, X = B and M = Mn(III).22 But, so far and so forth, none of the process could have been scaled up largely because either low selectivity of styrene oxide/benzaldehyde, or use of high temperature, or serious leaching of the catalyst. In our previous paper, we reported ∼52% yield of styrene oxide;23 but the catalyst shows inefficiency from its 3rd recycle and suffers severe leaching in the reaction medium. Therefore, a true heterogeneous catalyst (devoid of leaching properties) with high selective nature is highly demanding in the field of catalysis. The heterogeneous epoxidation of olefins by silver-based catalysts is an important process in chemical technologies,16,24 and the catalytic properties of AgNPs in oxidation reactions strongly depend on the particles' size and stability and the nature of the support.
Herein, we also report a styrene conversion of 75% with a styrene-oxide selectivity of 65% and a styrene conversion of >99% with a benzaldehyde selectivity of 88%, varying reaction parameters.
The Ag–WO3 aloevera catalyst was synthesized by modifying our own preparation method25 taking tungstic acid and silver nitrate as the precursors of W and Ag respectively. The evolution of silver supported tungsten oxide aloevera-like structure is really interesting although the mechanism is not very clear. The cationic surfactant CTAB, synthesis-stirring time nucleation-growth rate of the seed plays an important role in determining the morphology of the final nanoparticle. As per LaMer plot26 for the crystallization nucleation growth process and Tran's point, the nucleation rate increases with decreasing surface energy. The surfactant can affect surface energy and thus control the nucleation rate. According to Gibbs–Wulff theory, the equilibrium shape of a crystal is one that minimizes the surface energy for a given enclosed volume. If the surface energy is isotropic, the equilibrium shape will be spherical as the sphere has the minimum surface area. Inorganic nanoparticles often lead to spherical particles as this represents the lowest possible surface energy. 1D nanostructures is generated, if the surface energy is anisotropic.26 To explore the formation mechanism of Ag–WO3 aloevera, a series of time-dependent experiments were performed. In the absence of CTAB, disperse rods with indefinite aspect ratio were obtained (Fig. S1, ESI†). However, inhomogeneous rod-like nanostructures with small diameters assembled by disordered aspect ratio were obtained while Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CTAB molar ratio was 1
CTAB molar ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S2, ESI†). This experimental finding reflected the structure-directive property of CTAB. When the mixture was kept for 1 h aging, followed by calcinations, the product so obtained displayed that numerous nanoparticles assembled together and in a definite direction, the growth of the assembly occurred (shown by red arrows, Fig. S3, ESI†). 3 h aging time was proved to be optimum condition for the growth of the aloevera-type morphology. The SEM image of the sample displayed that almost homogeneously formed nanorods (1D) with diameter ∼70 nm grew in a definite direction and displayed aloevera-like morphology (Fig. S4, ESI†). While the aging time was prolonged to 10 h, these nanorods fused together by means of Ostwald ripening process and produced larger, indefinite-shaped aggregates (Fig. S5, ESI†).
1 (Fig. S2, ESI†). This experimental finding reflected the structure-directive property of CTAB. When the mixture was kept for 1 h aging, followed by calcinations, the product so obtained displayed that numerous nanoparticles assembled together and in a definite direction, the growth of the assembly occurred (shown by red arrows, Fig. S3, ESI†). 3 h aging time was proved to be optimum condition for the growth of the aloevera-type morphology. The SEM image of the sample displayed that almost homogeneously formed nanorods (1D) with diameter ∼70 nm grew in a definite direction and displayed aloevera-like morphology (Fig. S4, ESI†). While the aging time was prolonged to 10 h, these nanorods fused together by means of Ostwald ripening process and produced larger, indefinite-shaped aggregates (Fig. S5, ESI†).
As a cationic surfactant, CTAB is an ionic compound that ionized completely in water: quantities of anions OH−, WO42−, and Ag and cations CTA+, Ag+ existed in basic solution.27 Therefore, cooperative self-assembly between ionic CTAB molecules and charged species is built via electrostatic interaction in reaction solution. The formation of nearly spherical aggregates of nanoparticles after 3 h aging time may be brought from the strong electrostatic attraction between positive CTA+ cations and negative OH− anions on the surface of nanoparticles as well as the hydrophobic interactions and van der Waals attraction caused by adjacent CTAB adsorbing on Ag–WO3 nanoparticles. All these factors contribute greatly for the generation of aloevera-like morphology of the sample. We also observed that, without the use of AgNO3, and even change in the precursors of Ag–W, however, aloevera-type morphology was not generated (Fig. S6–S8, ESI†). Therefore, we tentatively, suggest that, CTAB-micelles in the solution, precursors of Ag, W and even aging time directly take part in the shape-controlled synthesis of Ag–WO3 aloevera.
The Ag–WO3 aloevera like catalyst was characterized thoroughly by XRD, XPS, SEM, TEM, Raman, FTIR, TGA, BET surface area and ICP-AES. The crystal structure and phase purity of the Ag–W aloevera-like catalyst were analysed by X-ray diffraction (XRD) The X-ray diffraction (XRD) pattern of the catalyst showed the peaks at 2θ values of 23.1°, 23.7°, 24.4°, 33.3° and 34.0°, confirm the formation of monoclinic WO3 (JCPDS no. 43-1035, space group: P21/n) (Fig. 1). The very small peaks at 2θ values at 44°, 64.3° correspond to metallic Ag crystal faces (200) and (220) respectively [JCPDS no. 04-0783]. No other peak due to other phase of tungsten-oxide was observed, indicating a useful method to synthesize high-purity under the present experimental conditions. XPS was utilized to detect the surface composition and the chemical state of the catalyst. X-ray photoelectron spectroscopy (XPS) analyses confirmed the presence of metallic silver (Ag) in the fresh sample from the corresponding Ag 3d5/2 and Ag 3d3/2 binding energy values of 368.2 eV and 374.2 eV respectively (Fig. S9, ESI†).28 The W 4f5/2 and 4f7/2 spectra attributed to the binding energies 37.9 eV and 35.8 eV respectively suggesting that the tungsten in the tungsten oxide sample exists as W6+ (Fig. S10, ESI†).29 The corresponding Ag 3d binding energy of the spent catalyst ∼368.2 eV, confirms that the oxidation state of metallic silver does not change during catalysis.
 | ||
| Fig. 1 XRD diffractogram of (a) fresh catalyst, (b) spent catalyst (after 4 recycles), commercial (c) Ag and (d) WO3. | ||
The topology of the catalyst was studied by scanning electron microscopy (SEM, Fig. 2 & S4, ESI†). SEM images with lower magnification (Fig. 2a) revealed that the catalyst displays aloevera-like structure. The oriented texture of the sample can be better seen from much lower magnification (Fig. S4, ESI†). A high magnification image of the catalyst (Fig. 2c) reveals that most of the nanorods are oriented and have a uniform diameter and length. It also seems from the diagram that Ag nano particles are visualized like water-droplets on aloevera-leaves. SEM-EDX analysis (Fig. 2d) of the composite revealed that, there appears a distribution of Ag, W and O only, and no sort of C or Br. This observation indicated the complete removal of the structure-directing template(s). However, SEM-EDX analysis of the uncalcined composite revealed the presence of C and N as impurity (marked as blue circle, Fig. S11, ESI†). This experimental finding was further supported from TGA (Fig. 3) and FTIR analysis of the uncalcined catalyst (Fig. 4). TGA analyses (Fig. 3) were carried out to understand the various weight-loss regimes of the uncalcined catalyst. Three discrete regions of thermal decomposition can be observed; first weight-loss corresponds to the elimination of water followed by the decomposition of reactants to form NOx and organic phases at 150 to 230 °C and finally the combustion process between 250 and 330 °C. A further mass loss is noticed between 350 and 450 °C due to the elimination of remaining carbon and organic compounds. This region is likely due to formation of a crystallized Ag–W–O inorganic phase. Further weight loss was not observed when the temperature was further increased from 480 to 600 °C, no weight loss speculated, indicating the formation of stable Ag–WO3 catalyst in that temperature zone.
 | ||
| Fig. 2 SEM images (a–c) in increasing magnifications and (d) SEM-EDAX of Ag–WO3 aloevera-type catalyst. | ||
 | ||
| Fig. 4 FTIR diagram of (a) fresh and (b) spent (after 5 reuses), (c) uncalcined Ag–WO3 aloevera-type catalyst and (d) that of CTAB. | ||
The embedment of CTAB molecules on the uncalcined catalyst was further confirmed from the FTIR analysis (Fig. 4). The peaks of the sample at 812, 1062 cm−1 can be assigned to the C–N+ stretching modes of CTAB molecules.30 The peak at 1378 and at 1462 cm−1 are assigned to symmetric mode of vibration of the head groups of the methylene moiety (N+–CH3) and CH2 scissoring mode respectively.30 The frequencies above 1600 cm−1 to 3000 cm−1 are due to CH2 symmetric and antisymmetric vibrations, respectively. It is to be noted that, the shift of vibrations to lower frequency occurred as the alkyl chains experienced a more hydrophobic environment in Ag–W blocks upon the surface of which the CTA-moieties were supposed to be bound.2 It can be inferred that, the mutual interactions between CTAB and the Ag–W surface have taken place. Some bands were detected at 2800–3020 cm−1, which can be attributed to the CTAB surfactant. The FTIR spectrum of CTAB shows two intense bands at 2918 and 2846 cm−1, corresponding to the asymmetric and symmetric stretching vibrations of C–CH in the methylene chains. The sharp bands at 1450–1500 cm−1 were specified as the deformation of –CH2– and –CH, and the weak band at 3011 cm−1 as the C–CH3 asymmetric stretching and N–CH symmetric stretching vibrations of the solid surfactant.30 These typical frequencies were absent when the material was calcined at 500 °C in air (fresh catalyst) in the case of the prepared catalyst, which indicated that, the embedded CTAB moieties have been completely removed from the catalyst surface during calcination. In the crystalline structure of WO3, W atoms are located in the centre of WO6 octahedra with O at the vertices forming W–O–W connections.31 For such an arrangement, the IR active bands are fundamental vibrations of W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, W–O and W–O–W. These can be stretching (ν) or in-plane bending vibrations (δ) and out-of-plane wagging (γ).31 The main WO3 vibrations are found in the 1700–380 cm−1 IR region. All synthesis temperatures present characteristic peaks at 570, 800, 895, 964, 1045, 1404 and 1608 cm−1 associated with γ(W–O–W), ν(W–O–W), ν(W–O–W), ν(W–O, W
O, W–O and W–O–W. These can be stretching (ν) or in-plane bending vibrations (δ) and out-of-plane wagging (γ).31 The main WO3 vibrations are found in the 1700–380 cm−1 IR region. All synthesis temperatures present characteristic peaks at 570, 800, 895, 964, 1045, 1404 and 1608 cm−1 associated with γ(W–O–W), ν(W–O–W), ν(W–O–W), ν(W–O, W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), δ(W–OH), ν(OH) and δ(OH) in W–OH respectively. The –OH bands are associated with surface hydroxyl groups and weakly bound solvent, ethanol–water. A stronger broad band around 3350 cm−1 should appear from a W–OH mode if intercalation of H2O had occurred.31 Moreover, there was no structural deformation in the spent catalyst, that was observed from FTIR analysis.
O), δ(W–OH), ν(OH) and δ(OH) in W–OH respectively. The –OH bands are associated with surface hydroxyl groups and weakly bound solvent, ethanol–water. A stronger broad band around 3350 cm−1 should appear from a W–OH mode if intercalation of H2O had occurred.31 Moreover, there was no structural deformation in the spent catalyst, that was observed from FTIR analysis.
To further investigate the surface property and to detect subtle phase information of the composite, we conducted Raman-spectrum analysis (Fig. 5). Raman spectra of the Ag–WO3 flower catalyst is characterized by well resolved sharp bands as shown. The two main intense peaks at 806 and 718 cm−1, are typical Raman peaks of crystalline WO3, which correspond to the stretching and bending vibrations of the bridging tungsten and oxygen atoms. They are assigned to be the W–O stretching (ν), W–O bending (δ) and O–W–O deformation (γ) modes, respectively. Two peaks at 326 and 274 cm−1 are assigned to be the bending δ(O–W–O) vibrations.31 Those below 200 cm−1 modes were attributed to the lattice vibrations.31 After the reaction, the Raman spectrum of the spent catalyst was unchanged, reflects the structural stability of the catalyst under the reaction condition.
TEM was used to further investigate the sizes and shapes of the catalyst (Fig. 6). From the TEM image, it could be concluded that this preparation method has successfully overcome the problem of agglomeration and appropriate dispersion to obtain nanorods with uniform size. TEM measurements were carried out to check the particle size and distribution of the silver nanoparticles (Fig. 6b, inset, based on Fig. 6b). Higher magnification of the catalyst revealed that the catalyst is composed of highly dispersed very small silver nanoparticles of ∼2–5 nm on WO3 support (Fig. 6a and b). The corresponding TEM histogram of Ag nanoparticles showed a very narrow particle size distribution with sizes between 2.5–6.5 nm (Fig. 6b, inset). The interplanar spacing of the lattice fringe distance of 0.38 nm indicates the [020] lattice spacing of WO3, which was clearly discriminated from of 0.23 nm corresponds to [111] plane of Ag (Fig. 6d).25 Additionally, the SAED pattern (Fig. 6c) also displayed a polycrystalline nature of the aloevera structure, indicating that the AgW rods are randomly orientated. Furthermore, the TEM image of the spent catalyst showed that the topology and the silver particle size of the catalyst were hardly changed after fiver reuses (Fig. S12, ESI†). TEM-EDX pattern also showed the presence of Ag and W in the sample (Fig. S14a, ESI†). Moreover, that the percentage of Ag and W remain intact after four reuses qualitatively is also visualized from the corresponding TEM-EDAX image of the spent catalyst (Fig. S14b, ESI†). It was also visualized from TEM-images (Fig. S15, ESI†), that increment in Ag-loading led to the increase in Ag-particle size. Moreover, some agglomerations of Ag-particles were also observed from the TEM diagram.
 | ||
| Fig. 6 TEM images (a and b) with increasing magnifications, (c) SAED pattern and (d) HRTEM image of the Ag–WO3 aloevera-like catalyst. | ||
Typically, the dispersion of Ag, W and O atoms in the catalyst was also analyzed by STEM-EDX mapping (Fig. 7). It indicated that each of Ag, W and O species was homogeneously dispersed.
 | ||
| Fig. 7 (a) STEM image and elemental distribution (based on (a)) of (b) Ag, (c) W and (d) O in the Ag–WO3 aloevera-like catalyst. | ||
The activities of Ag–WO3 aloevera (Ag–Walv) catalyst in the oxidation of styrene in liquid phase by using H2O2 as oxidant have been summarized in Table 1. Main product was detected to be styrene oxide (SO). Main by-products were detected to be benzaldehyde (ΦCHO), phenylacetaldehyde (ΦCH2CHO) and very less amount of benzoic acid (ΦCOOH). Oxidation of styrene was speculated to occur in a single pathway i.e. solely in the side chain, attached with aromatic system and none in the aromatic ring. At room temperature (35 °C), the poor conversion of styrene conversion was noticed. At higher temperatures (>50 °C) although conversion of styrene increased, selectivity to phenylacetaldehyde was noticed to be sharply dropping due to the formation of mainly benzaldehyde (Fig. S16, ESI†). To maintain the higher selectivity of SO, we tried to carry out the reactions at moderate temperature (at 75 °C), where we also expected the satisfied conversion of styrene (75% conversion). Then we varied different reaction conditions to achieve higher yield of SO at 75 °C. When styrene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 molar ratio was low (say 1
H2O2 molar ratio was low (say 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), we observed low conversion of styrene (Fig. S17, ESI†) probably due to inevitable decomposition of H2O2 at that temperature over the catalyst. Blank reaction was performed (entry 1, Table 1 & Fig. S18, ESI†) without any catalyst; conversion of styrene was very poor, reflecting the necessity of the catalyst in this oxidation reaction. We also observed that, increment in catalyst weight decreased the selectivity of acetophenone (Fig. S18, ESI†), probably due to increase of more active catalytic sites facilitated the attacking positions of styrene in its all possible sites. Maintaining all the optimum conditions, when the reaction was allowed to run for hours (Fig. S19, ESI†), we noticed that, although the conversion of styrene increased with time, but SO selectivity gradually went decreasing due to the formation benzaldehyde and other by-products.
2), we observed low conversion of styrene (Fig. S17, ESI†) probably due to inevitable decomposition of H2O2 at that temperature over the catalyst. Blank reaction was performed (entry 1, Table 1 & Fig. S18, ESI†) without any catalyst; conversion of styrene was very poor, reflecting the necessity of the catalyst in this oxidation reaction. We also observed that, increment in catalyst weight decreased the selectivity of acetophenone (Fig. S18, ESI†), probably due to increase of more active catalytic sites facilitated the attacking positions of styrene in its all possible sites. Maintaining all the optimum conditions, when the reaction was allowed to run for hours (Fig. S19, ESI†), we noticed that, although the conversion of styrene increased with time, but SO selectivity gradually went decreasing due to the formation benzaldehyde and other by-products.
| Entry | Catalyst | C S b (%) | S P c (%) | Y SO d (%) | E 0 e | |||
|---|---|---|---|---|---|---|---|---|
| SO | Φ CHO | Φ CH2CHO | Φ COOH | |||||
a Typical reaction conditions: solvent (MeCN) = 10 ml, substrate (styrene) = 1 g, catalyst = 0.075 g, styrene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 (molar ratio) = 1 H2O2 (molar ratio) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, reaction temperature = 75 °C; time = 12 h.
b
C
S = conversion of styrene based upon the FID-GC using methanol as external standard = [moles of styrene reacted/initial moles of styrene used] × 100.
c
S
P = selectivity to SO = [moles of products produced/moles of styrene reacted] × 100.
d
Y
SO = yield of SO = CS × SSO/100.
e
E
0 = H2O2 efficiency = [moles of SO or benzaldehyde formed/total moles of H2O2 added] × 100.
f Prepared Ag–WO3 aloevera catalyst.
g Catalyst after 5 reuse.
h Styrene 4, reaction temperature = 75 °C; time = 12 h.
b
C
S = conversion of styrene based upon the FID-GC using methanol as external standard = [moles of styrene reacted/initial moles of styrene used] × 100.
c
S
P = selectivity to SO = [moles of products produced/moles of styrene reacted] × 100.
d
Y
SO = yield of SO = CS × SSO/100.
e
E
0 = H2O2 efficiency = [moles of SO or benzaldehyde formed/total moles of H2O2 added] × 100.
f Prepared Ag–WO3 aloevera catalyst.
g Catalyst after 5 reuse.
h Styrene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 (molar ratio) = 1 H2O2 (molar ratio) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.
i Ag loading = 5.8%; COM: commercial; IMP: impregnation method; alv: aloevera-like; loading of Ag was determined from ICP-AES. 6.
i Ag loading = 5.8%; COM: commercial; IMP: impregnation method; alv: aloevera-like; loading of Ag was determined from ICP-AES.
|
||||||||
| 1 | No catalyst | 1.5 | 35 | 45 | 17 | 3 | 0.5 | — |
| 2 | Ag | 1.8 | 18 | 48 | 30.5 | 3.5 | 0.3 | — |
| 3 | Ag2O | 2.2 | 14 | 46.5 | 35 | 4.5 | 0.3 | — |
| 4 | WO3 | 2.8 | 22 | 52 | 20.5 | 5.5 | 0.6 | — |
| 5 | Ag–Wimp | 2.5 | 26 | 56 | 15 | 3 | 0.6 | — |
| 6f | Ag–Walv3.3 | 75 | 55 | 43 | 2 | 1 | 41.2 | 10.3 |
| 7g | Ag–Walv3.3 | 72.5 | 52 | 44 | 2.5 | 1.5 | 37.7 | 9.4 |
| 8h | Ag–Walv3.3 | >99.0 | 5 | 88 | 3 | 4 | 5 | 14.5 |
| 9i | Ag–Walv5.8 | >99.0 | 27 | 67 | 3 | 2 | 1 | 16.5 |
Notably, commercial Ag2O, WO3, and eve metallic Ag catalyst did not show any activity (entry 2–4, Table 1). Conventional catalyst prepared by impregnation method also showed negligible activity (entry 5, Table 1). The reason can be attributed to the comparatively smaller size of Ag (supported) nanoparticles catalyst possess comparatively high specific surface area which corresponds to higher dispersion of the catalyst that leads to the availability of more exposed surface active sites, where the catalytic reaction takes place. The open structure of the WO3 nanorods allows easy access of the reactants which makes the catalytic process favorable. The poor catalytic activity of the impregnated catalyst may be attributed to their irregular shape and larger particles size which limits the accessibility of the catalyst towards the reacting substrates (Fig. S13, ESI†).
We also noticed that, further increment in content of Ag to 5.8%, however, the catalytic efficiency decreased (entry 9, Table 1). This may be attributed to the slightly aggregation of the Ag-NPs on the surface of WO3 nanorods, resulting in the decrease of the number of active sites on the sample of the catalyst.
The optimum reaction condition of the oxidative coupling reaction was applied on various substituted styrenes (Table 2). Epoxides were the main product; moreover, we noticed that, electron-donating group facilitates the epoxidation reaction, whereas electron-withdrawing group retards it. From this experimental finding, it can be concluded that, the mechanism follows a radical-formation mechanism pathway.
| Entry | Substrates | Main product | C s (%) | S SO (%) | Y SO (%) |
|---|---|---|---|---|---|
a Typical reaction conditions: solvent (MeCN) = 10 ml, substrate = 1 g, catalyst = 0.075 g, substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 (molar ratio) = 1 H2O2 (molar ratio) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, reaction temperature = 75 °C; time = 12 h. 4, reaction temperature = 75 °C; time = 12 h.
|
|||||
| 1 |

|

|
78 | 48 | 37.4 |
| 2 |

|
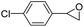
|
55 | 37 | 20.3 |
| 3 |
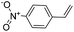
|

|
18 | 27 | 4.9 |
The H2O2 does not interact with styrene under normal conditions. H2O2 dissociation is believed to occur homogeneously over the dissociation of H2O2 over Ag–WO3 generates the peroxo tungsten species which behave as an electrophile in this reaction and the electrophilic oxygen attacks the electron dense C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond; thereby SO moiety is produced on the surface of the catalyst.32 Hampering the optimum condition, i.e. use of excess H2O2 or use of higher temperature led to the rupture of 3-membered epoxy-system (SO) and produces more stable product benzaldehyde.
C bond; thereby SO moiety is produced on the surface of the catalyst.32 Hampering the optimum condition, i.e. use of excess H2O2 or use of higher temperature led to the rupture of 3-membered epoxy-system (SO) and produces more stable product benzaldehyde.
The oxidant H2O2 was used in an excess amount (styrene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 1
H2O2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratio). In general, H2O2 decomposes spontaneously over a catalytic surface. Hence, we used an excess of H2O2 so that the active oxygen species needed for the oxidation of the styrene could be available during the reaction. Significant H2O2 decomposition occurs on the catalyst necessitating its usage in three-fold excess. Furthermore, we took the reaction mixture and performed permanganometric titrations to detect H2O2, but no H2O2 was discovered in the reaction mixture, indicating that the unreacted H2O2 molecules have been decomposed completely. We have plotted H2O2 consumption in terms of its efficiency% (E0) in Table 1.
4 molar ratio). In general, H2O2 decomposes spontaneously over a catalytic surface. Hence, we used an excess of H2O2 so that the active oxygen species needed for the oxidation of the styrene could be available during the reaction. Significant H2O2 decomposition occurs on the catalyst necessitating its usage in three-fold excess. Furthermore, we took the reaction mixture and performed permanganometric titrations to detect H2O2, but no H2O2 was discovered in the reaction mixture, indicating that the unreacted H2O2 molecules have been decomposed completely. We have plotted H2O2 consumption in terms of its efficiency% (E0) in Table 1.
The efficiency of a heterogeneous catalyst is evaluated in terms of its recyclability and stability. The reusability of the Ag–WO3 aloevera catalyst was studied without any regeneration. After each run, the catalyst was filtered during hot condition and repeatedly washed with acetonitrile and acetone and dried overnight at 100 °C and used as such. We observed that the catalyst showed negligible change in its activity (entry 7, Table 1 & Fig. S20, ESI†). The amount of Ag and W present in the catalyst after 5 reuse was almost same as the fresh catalyst (estimated by ICP-AES) confirming the true heterogeneity of the catalyst. After 5 recycles, negligible amount of leaching of Ag and W was detected by ICP-AES (concentration of both metals were <2 ppb).
Conclusions
In summary, we have developed a surfactant-promoted simple preparation method to prepare Ag–WO3 aloevera like catalyst, comprising 2.5–6.5 nm Ag nanoparticles anchored on WO3 rods with ∼70 nm diameter, displaying high thermal stability and good catalytic activity for the single step conversion of styrene to styrene oxide/benzaldehyde using H2O2, exhibiting a styrene conversion of 75% with a styrene-oxide selectivity of 55% and a styrene conversion of >99% with a benzaldehyde selectivity of 88% varying different reaction conditions. The catalyst can be reused several times without any activity loss. The proposed method is also advantageous from the standpoint of low cost, environmental benignity and operational simplicity; furthermore, it can be applicable to large-scale reactions.Acknowledgements
SG and SSA thank UGC and CSIR, New Delhi, India, for their respective fellowships. R.B. thanks CSIR, New Delhi, for financial support in the form of the 12 FYP Project (CSC-0125, CSC-0117). The Director, CSIR-IIP is acknowledged for his help and encouragement. The authors thank ASD Division, IIP for the analytical services. We gratefully acknowledge Ms Nishita Lucas, CSIR-NCL, Pune (India), for performing Raman spectroscopy analyses.Notes and references
- C. L. Jiang, F. Wang, N. Q. Wu and X. G. Liu, Adv. Mater., 2008, 20, 4826–4829 CrossRef CAS PubMed.
- Y. Cui, Q. Wei, H. Park and C. M. Lieber, Science, 2001, 293, 1289–1292 CrossRef CAS PubMed.
- C. L. Jiang, S. Ranjit, Z. Y. Duan, Y. L. Zhong, K. P. Loh, C. Zhang and X. G. Liu, Nanoscale, 2009, 1, 391–394 RSC.
- W. Lueangchaichaweng, N. R. Brooks, S. Fiorilli, E. Gobechiya, K. Lin, L. Li, S. Parres-Esclapez, E. Javon, S. Bals, G. V. Tendeloo, J. A. Martens, C. E. A. Kirschhock, P. A. Jacobs and P. P. Pescarmona, Angew. Chem., 2014, 53, 1585–1589 CrossRef CAS PubMed.
- L. Z. Wang, J. L. Zhang, F. Chen and M. Anpo, J. Phys. Chem. C, 2007, 111, 13648–13651 CAS.
- L. P. Zhu, W. D. Zhang, H. M. Xiao, Y. Yang and S. Y. Fu, J. Phys. Chem. C, 2008, 112, 10073–10078 CAS.
- K. Kamata, K. Yonehara, Y. Nakagawa, K. Uehara and N. Mizuno, Nat. Chem., 2010, 2, 478–483 CrossRef CAS PubMed.
- C. Jia, T. Kitamura and T. Fujiwara, Acc. Chem. Res., 2001, 34, 633–639 CrossRef CAS PubMed.
- S. S. Acharyya, S. Ghosh, R. Tiwari, B. Sarkar, R. K. Singha, C. Pendem, T. Sasaki and R. Bal, Green Chem., 2014, 16, 2500–2508 RSC.
- D. Swern, Organic Peroxide, Wiley Interscience, New York, 1971 Search PubMed.
- M. J. Rak, M. Lerro and A. Moores, Chem. Commun., 2014, 50, 12482–12485 RSC.
- F. Brühne and E. Wright, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000, DOI:10.1002/14356007.a03_463.
- J. Liu, F. Wang, S. Qi, Z. Gu and G. Wu, New J. Chem., 2013, 37, 769–774 RSC.
- S. Das, A. Goswami, M. HesarI, J. F. Al-Sharab, E. K. Mikmeková, F. Maran and T. Asefa, Small, 2014, 10, 1473–1478 CrossRef CAS PubMed.
- B. Singh and A. K. Sinha, J. Mater. Chem. A, 2014, 2, 1930–1939 CAS.
- X. Liu, A. Klust, R. J. Madix and C. M. Friend, J. Phys. Chem. C, 2007, 111, 3675–3679 CAS.
- A. Dhakshinamoorthy, A. Primo, P. Concepcion, M. Alvaro and H. Garcia, Chem.–Eur. J., 2013, 19, 7547–7554 CrossRef CAS PubMed.
- S. Sharma, S. Sinha and S. Chand, Ind. Eng. Chem. Res., 2012, 51, 8806–8814 CrossRef CAS.
- K. Kamata, K. Yonehara, Y. Sumida, K. Yamaguchi, S. Hikichi and N. Mizuno, Science, 2003, 300, 964–966 CrossRef CAS PubMed.
- S. C. Laha and R. Kumar, J. Catal., 2001, 204, 64–70 CrossRef CAS.
- F. Rajabi, N. Karimi, M. R. Saidi, A. Primo, R. S. Varma and R. Luque, Adv. Synth. Catal., 2012, 354, 1707–1711 CrossRef CAS PubMed.
- T. A. G. Duarte, A. C. Estrada, M. M. Q. Simões, I. C. M. S. Santos, A. M. V. Cavaleiro, A. G. P. M. S. Neves and J. A. S. Cavaleiro, Catal. Sci. Technol., 2015, 5, 351–363 CAS.
- B. Sarkar, R. K. Singha, R. Tiwari, S. Ghosh, S. S. Acharyya, C. Pendem, L. N. S. Konathala and R. Bal, RSC Adv., 2014, 4, 5453–5456 RSC.
- J. G. Serafin, A. C. Liu and S. R. Seyedmonir, J. Mol. Catal. A: Chem., 1998, 131, 157–168 CrossRef CAS.
- (a) S. Ghosh, S. S. Acharyya and R. Bal, J. Mater. Chem. A, 2014, 2, 15726–15733 RSC; (b) S. Ghosh, S. S. Acharyya, T. Sasaki and R. Bal, Green Chem., 2015, 17, 1867–1876 RSC.
- Z. Jiang, J. Xie, D. Jiang, X. Wei and M. Chen, CrystEngComm, 2013, 15, 560–569 RSC.
- C. L. Kuo and K. C. Hwang, Langmuir, 2012, 28, 3722–3729 CrossRef CAS PubMed.
- D. C. Lim, I. Lopez-Salido and Y. D. Kim, Surf. Sci., 2005, 598, 96–103 CrossRef CAS PubMed.
- N. Wang, D. Wang, M. Li, J. Shi and C. Li, Nanoscale, 2014, 6, 2061–2066 RSC.
- S. S. Acharyya, S. Ghosh and R. Bal, ACS Appl. Mater. Interfaces, 2014, 6, 14451–14459 CAS.
- O. Yayapao, T. Thongtem, A. Phuruangrat and S. Thongtem, J. Alloys Compd., 2011, 509, 2294–2299 CrossRef CAS PubMed.
- R. A. Sheldon and J. A. Van Doorn, J. Catal., 1973, 31, 427–437 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Catalyst preparation, catalyst-characterization techniques, SEM diagrams, XPS, effects of different reaction parameters on styrene oxidation etc. See DOI: 10.1039/c5ra03803k |
| This journal is © The Royal Society of Chemistry 2015 |


