Hydroxyl functionalization improves the surface passivation of nanostructured silicon solar cells degraded by epitaxial regrowth†
Abstract
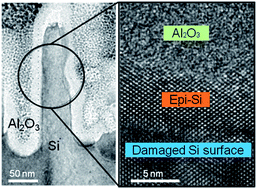
Metal-assisted chemical etching is useful and cost-efficient for nanostructuring the surface of crystalline silicon solar cells. We have found that the nanoscale epitaxy of silicon occurs, upon subsequent annealing, at the Al2O3/Si interface amorphized by metal-assisted etching. Since this epitaxial growth penetrates into the pre-formed Al2O3 film, the bonding nature at the newly formed interfaces (by the regrown epitaxy) is deteriorated, resulting in a poor performance of Al2O3 passivation. Compared to the conventional hydrogen (H–) passivation, hydroxyl functionalization by oxygen plasma treatment was more effective as the wafer became thinner. For ultrathin (∼50 μm) wafers, ∼30% depression in surface recombination velocity led to the improvement of ∼15.6% in the short circuit current. The effectiveness of hydroxyl passivation validated by ultrathin wafers would be beneficial for further reducing the wafer cost of nanostructured silicon solar cells.

 Please wait while we load your content...
Please wait while we load your content...