Structural, dielectric and magnetic investigations on Al3+ substituted Zn-ferrospinels
Abstract
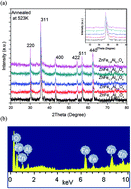
A series of ZnAlxFe2−xO4 (0.1 ≤ x ≤ 0.5) ferrospinels has been prepared by chemical co-precipitation method to understand the effect of Al3+ substitution on the structural, dielectric and magnetic response of ZnFe2O4 nanoparticles. X-ray diffraction (XRD) and transmission electron microscopy (TEM) images confirmed the nano size formation of particles. The lattice parameter (a), X-ray density (ρx) and bulk density (ρm) were found to decrease with increasing inclusion of Al3+ ions. AC conductivity (σac) measurements as a function of temperature show that the samples behave like semiconductors. Decrease in the hopping conduction between Fe2+ ↔ Fe3+ ions at octahedral site is observed with increasing inclusion of Al3+ ions. The Nyquist plots of the prepared materials reveal the inherent phenomenon involved in conduction mechanism of Al3+ substituted ZnFe2O4 ferrites. The magnetization studies revealed that magnetic moment (ηB) showed decreasing trend with increase in substitution of Al3+, its value decreases from 0.56 (for x = 0.1) to 0.34 (for x = 0.5). The Ms values decrease from 13.29 emu g−1 for x = 0.1 to 8.42 emu g−1 for x = 0.5. DM (magnetic particle size) was found to be less than the particle size calculated from TEM micrographs due to presence of magnetically dead layer on the surface of particle. Squareness (S) values infer that particles interact by magnetostatic interactions. The M − H loop of all the samples is narrow with low value of coercivity and retentivity; indicates the superparamagnetic nature of prepared nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...