Facile synthesis of γ-Fe2O3 nanoparticles integrated H2Ti3O7 nanotubes structure as a magnetically recyclable dye-removal catalyst†
Abstract
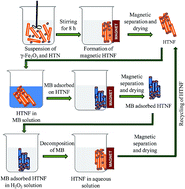
Nanocomposites consisting of γ-Fe2O3 nanoparticles incorporated in hydrogen titanate (H2Ti3O7 or lepidocrocite-type or H2Ti2O4(OH)-type structures) nanotubes (HTNF) have been synthesized through a simple strategy involving the combination of hydrothermal treatment followed by an ion-exchange process both conducted in aqueous media. The resulting nanocomposites reveal high efficiency in dye-adsorption capacity, magnetic separability from an aqueous solution, and recyclability. The unique nanostructures of HTNF composites are composed of γ-Fe2O3 nanoparticles typically attached to the ends of HTN bundles rather than along the surface and exhibit high magnetic separability in an aqueous medium using a moderate external magnetic field. The methylene blue (MB) dye-adsorption characteristics of HTNF nanocomposites have been investigated by varying the amount of γ-Fe2O3 (0–25 wt%) and the initial MB concentration (∼7.5–250 μM) at the initial solution-pH of ∼10. The HTNF nanocomposite with 5 wt% γ-Fe2O3 shows relatively higher MB dye adsorption capacity (99 mg g−1) along with reasonable magnetic separability (2 min) from an aqueous solution. The MB adsorption on the surface of the HTNF nanocomposites follows a pseudo-second-order kinetics model and the equilibrium adsorption isotherm follows both the Langmuir and Dubinin–Kaganer–Radushkevich (DKR) models. The recyclability of the HTNF magnetic nanocomposite in dye-removal application has been demonstrated by decomposing the previously adsorbed MB dye via surface-cleaning treatment conducted in H2O2 solution.

 Please wait while we load your content...
Please wait while we load your content...