Understanding the effect of interaction among aeration, agitation and impeller positions on mass transfer during pullulan fermentation by Aureobasidium pullulans†
Abstract
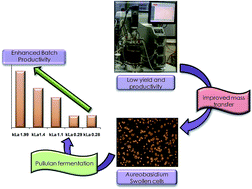
Pullulan is a non-ionic, water-soluble homopolysaccharide produced via fermentation using Aureobasidium pullulans, a black yeast. The unique physicochemical properties of pullulan have made it a potential candidate for a diverse range of applications in various industrial sectors such as food, pharmaceutical, cosmetics, and biomedicine. Low yield and productivity are major challenges for the extensive commercialization of this biopolymer, and they are often found to be associated with poor mass transfer during the fermentative production of pullulan. The present study is an attempt to address this challenge by a unique multivariate approach. The interactive influence of air-flow rates, agitation speed and impeller spacing on volumetric mass transfer coefficient (kLa) was investigated using a statistical design. The design has demonstrated a unique correlation between mass transfer capabilities and process variables, whereas pullulan fermentation kinetics has revealed a dependence of microbial activity on kLa values in a bioprocess. Furthermore, the resultant mass transfer conditions were applied for pullulan production, which enhanced the productivity significantly (1.61 g L−1 h−1). These observations might help in obtaining improved mass transfer conditions for the economic production of pullulan.

 Please wait while we load your content...
Please wait while we load your content...