Core–shell Co3O4/α-Fe2O3 heterostructure nanofibers with enhanced gas sensing properties†
Jing Caoab,
Ziying Wanga,
Rui Wanga,
Sen Liua,
Teng Feia,
Lijie Wanga and
Tong Zhang*ab
aState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, P. R. China. E-mail: zhangtong@jlu.edu.cn; Fax: +86 431 85168270; Tel: +86 431 85168385
bState Key Laboratory of Transducer Technology, Chinese Academy of Sciences, P. R. China
First published on 7th April 2015
Abstract
1D Co3O4/α-Fe2O3 core–shell nanofibers were successfully fabricated by a facile and template-free coaxial electrospinning method, and developed for acetone gas detection. Morphology and component characterizations confirm that the as-prepared 1D heterostructures possess a well-defined core–shell structure with Co3O4 in the core and α-Fe2O3 in the shell. The unique 1D core–shell nanostructures, heterojunction effect at the Co3O4/α-Fe2O3 interfaces and the catalysis of Co3O4 endow the Co3O4/α-Fe2O3 core–shell nanofiber-based sensor with an enhanced gas sensing performance in terms of good sensing selectivity, high sensitivity (11.7), rapid response/recovery times (2 s/20 s) and better reproducibility for acetone gas. The gas sensing mechanism is proposed in detail. Overall, the 1D Co3O4/α-Fe2O3 core–shell heterostructure nanofibers synthesized through coaxial electrospinning make a promising and effective acetone sensor.
1. Introduction
With increasing concerns about the effect of air pollution and noxious gas on human health and safety, highly effective oxide semiconductor gas sensors have attracted more and more attention due to their significant resistance change upon exposure to trace concentrations of reducing or oxidizing gases.1,2 In order to improve gas sensor performances, great efforts have been made in the design and engineering of novel materials.3–5 Among them, core–shell structured nanomaterials, which consist of chemically distinct components, have been widely investigated as one of the most promising materials for gas detection and have exhibited enhanced gas sensing properties compared to single oxides.6,7 Up to now, many fabrication techniques have been reported for the preparation of a variety of oxide semiconductor core–shell structures.8–12 Despite their success, many of these approaches usually involve high temperatures, complex operation steps or especial solvents and additives, which possibly result in increased expense and hence limit their potential applications. Therefore, it is of great necessity to find simpler and more dependable synthetic methods for core–shell nanostructures with designed chemical components and controlled morphologies.Co3O4, a typical p-type metal oxide semiconductor, has been widely studied as a promising gas sensing material.13,14 On the other hand, α-Fe2O3 is an important n-type semiconductor oxide and has been extensively investigated as a gas sensor.15,16 Furthermore, it has been reported that the combination of p- and n-type nanomaterials can provide higher and faster responses.17 Thus, the core–shell nanostructures between p-type Co3O4 and n-type α-Fe2O3 can provide a valuable material platform for high performance gas sensors. Although Co3O4 has been made into composites with various n-type semiconductors, very little work is directed toward fabricating Co3O4/α-Fe2O3 core–shell nanostructures and researching their gas sensing properties. Therefore, the combination of Co3O4 and α-Fe2O3 as a core–shell structure is of vital scientific and practical significance, and is expected to exhibit enhanced gas sensing properties.
In this work, 1D Co3O4/α-Fe2O3 core–shell heterostructure nanofibers were successfully fabricated by a simple coaxial electrospinning route combined with a subsequent calcination process. The present synthesis method for the core–shell structure is facile, economical and environmentally friendly. The sensors based on the as-prepared 1D Co3O4/α-Fe2O3 core–shell nanostructures exhibit significantly enhanced acetone gas sensing performance in terms of good sensing selectivity, high sensitivity, a rapid response/recovery time and better reproducibility, indicating that the as-prepared Co3O4/α-Fe2O3 core–shell nanostructures can be developed for acetone gas detection.
2. Experimental section
2.1 Setup for coaxial electrospinning and preparation of Co3O4/α-Fe2O3 core–shell nanofibers
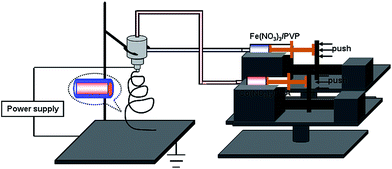
All of the reagents involved in the experiments were of analytical reagent grade and were directly used without further purification. As shown in Fig. 1, the Co3O4/α-Fe2O3 core–shell nanofibers were fabricated by a coaxial electrospinning method. Four main parts including a high voltage DC power supply, the fluid supply system, composite nozzles and the collecting board formed the setup for coaxial electrospinning. In particular, the fluid supply system was composed of a composite nozzle and thrust unit. The composite nozzle was assembled with two stainless pins in coaxial alignment and the diameters of the two stainless pins were different. In a typical procedure, an outer fluid was prepared by adding 0.6 g FeCl3 and 1.8 g PVP (polyvinyl pyrrolidone) in 10 mL DMF (N,N-dimethylformamide) to obtain aqueous solutions. A pale green solution was immediately produced under magnetic stirring for 2 minutes. An inner fluid was prepared by dissolving 1 g Co(NO3)2·6H2O in 15 mL PVA (polyvinyl alcohol) solution, which contained 15 mL of deionized water and 1 g PVA. The as-prepared precursor solutions were loaded into the syringes in the electrospinning setup. The fluid speeds of the inner and outer fluids were 0.7 and 0.8 mL h−1, respectively, and could be precisely controlled by the thrust unit. In the experiment, a voltage of 15 kV and a collection distance of 20 cm were employed for electrospinning. An ambient temperature and humidity were maintained at 24–27 °C and 30–40%, respectively, during the whole coaxial electrospinning procedure. The as-electrospun PVA/Co(NO3)2·9H2O core–PVP/FeCl3 shell composites were calcined at 600 °C for 1 h in an oxygen environment using a tube-type furnace to obtain the desired Co3O4/α-Fe2O3 core–shell nanofibers. The heating rate was controlled at 1 °C min−1.2.2 Fabrication and measurement of gas sensors
Fabrication of the sensors was similar to our previous work. Briefly, 0.01 g as-prepared Co3O4 nanofibers, α-Fe2O3 hollow nanofibers and Co3O4/α-Fe2O3 core–shell nanofibers were uniformly dispersed into 0.1 mL distilled water to form a paste. Then the paste was painted on the surface of a ceramic tube (outer diameter = 1.35 mm, length = 4 mm) on which a pair of gold electrodes was previously printed. A Ni–Cr heating wire was inserted in the tube to form a side-heated gas sensor. The prepared sensors were dried at 100 °C for 3 h and then welded on a socket for testing. Gas sensing properties of the sensor were measured by a RQ-2 series Intelligent Test Meter (China). The response (R) was defined using the formula R = Ra/Rg or R = Rg/Ra for the n-type and p-type oxide semiconductor, respectively. Ra and Rg were the resistance in the absence and presence of acetone gas, respectively. The response and recovery times (tres, trec) were defined as the time taken by the sensor to achieve 90% of the total resistance change upon exposure to the acetone gas and air, respectively.2.3 Characterization
Field emission scanning electron microscopy (FESEM) images were obtained using a JEOL JSM-6700F microscope. Transmission electron microscopy (TEM) and mapping images were obtained on a JEOL JEM-2200FS microscope operated at 200 kV. The crystal phases of the synthesized samples were characterized by X-ray powder diffraction (XRD, Rigaku D/max-Ra).3. Results and discussion
3.1 Morphology and composition analysis
The morphologies of the as-prepared Co3O4/α-Fe2O3 CSNFs, α-Fe2O3 HNFs and Co3O4 NFs were observed by field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). The low magnification FESEM image in Fig. 2a shows that the obtained Co3O4/α-Fe2O3 CSNFs are randomly oriented forming non-woven mats, and are uniform in the long axial direction with diameters of about 70–130 nm. The high magnification FESEM image in Fig. 2b clearly displays the cross section of the as-prepared core–shell structure and further confirms the distinctive bilayer structure consisting of the Co3O4 core and the α-Fe2O3 shell. Meanwhile, it can be found from Fig. 2b that the surfaces of the Co3O4/α-Fe2O3 CSNFs are rough due to the decomposition of the PVP polymer. Furthermore, α-Fe2O3 HNFs and Co3O4 NFs were also fabricated for comparison purposes. Fig. 2c and e show the low-magnification FESEM image of the α-Fe2O3 HNFs and the Co3O4 NFs respectively, in which both samples present long and continuous surfaces. The hollow structure of the α-Fe2O3 nanofibers can be easily observed by the cross-sectional image in Fig. 2d, while the enlarged FESEM image in Fig. 2f shows that the Co3O4 NFs are assembled by many small nanoparticles.Further morphology characterization of the Co3O4/α-Fe2O3 CSNFs, α-Fe2O3 HNFs and Co3O4 NFs was examined by TEM. Fig. 3a shows a typical TEM image of the Co3O4/α-Fe2O3 CSNFs. The core in shell structure can be obviously observed and the thickness of the shell layer in the core–shell nanofibers exhibits excellent uniformity. The TEM image is in good agreement with the FESEM result (Fig. 2b). As shown in Fig. 3b, the typical hollow morphology of the α-Fe2O3 HNFs is discerned by the TEM image, where the strong contrast difference between the darker marginal region and the brighter central region can be seen. In contrast, the TEM image from Fig. 3c demonstrates that the Co3O4 NFs are solid. Moreover, the Co3O4/α-Fe2O3 core–shell nanostructures were further characterized by an EDX element mapping test. Fig. 3d–f show the element mapping for Fe, Co and O respectively, in a single Co3O4/α-Fe2O3 core–shell nanofiber. The results demonstrate that the Fe and O are distributed throughout the core–shell nanofiber, whereas Co is concentrated at the core region, which again confirms the Co3O4-core/α-Fe2O3-shell structure. A similar phenomenon is also reported in the earlier literature.18,19 In addition, there is a small distribution of Co in the shell resin, because the boundary of PVP and PVA is not so distinct during the process of electrospinning, and a small quantity of Co2+ and Co3+ which dissolve in the PVA may diffuse to the shell layer during the heating process.
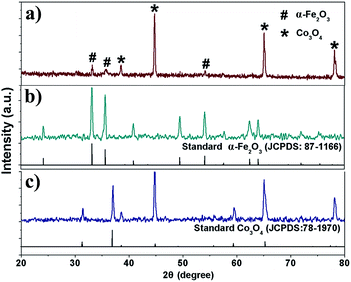
The structures of the as-prepared products were also characterized using XRD, which provided further insight into the crystallinity of the products. Fig. 4a shows the XRD pattern of the Co3O4/α-Fe2O3 CSNFs. All the diffraction peaks can be indexed to a mixture of α-Fe2O3 and Co3O4, which is consistent with the JCPDS file no. 87-1166 and 78-1970, respectively. No impurity phases were detected during the XRD measurements, which confirms the excellent purity of the products. Fig. 4b and c depict the XRD patterns of the as-prepared α-Fe2O3 (JCPDS no. 87-1166) HNFs and Co3O4 NFs (JCPDS no. 78-1970), respectively. No diffraction peaks from any other impurities were observed.
3.2 Acetone sensing properties
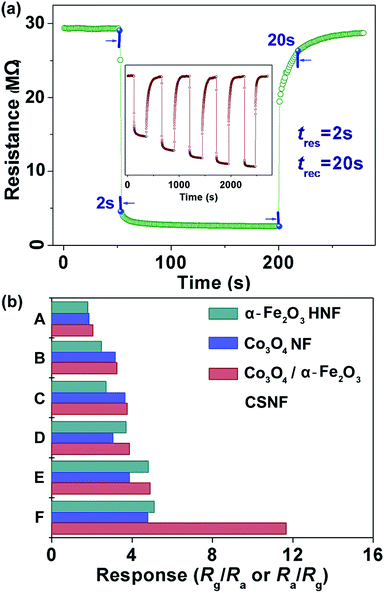
Acetone, as a common reagent, is widely used in industries and laboratories, and is harmful to human health and safety. Inhalation of acetone may cause headaches, fatigue, nausea and even narcosis and is harmful to the nervous system.20 On the other hand, acetone is also an important breath biomarker for noninvasive diagnosis of human diabetes.21 Hence, the detection of acetone is of great importance and much needed for both environmental protection and human health. Thus, a gas sensor based on the as-prepared 1D Co3O4/α-Fe2O3 core–shell nanostructures was fabricated and its acetone gas sensing performance were investigated.In gas sensing studies, the operating temperature is important for the investigation of gas sensing properties due to its great influence on the surface state of sensing materials, as well as the contact reactions during the gas sensing process.22 To investigate the influence of the operating temperature and to obtain an optimum operating temperature for the sensors, the responses of the Co3O4/α-Fe2O3 CSNF, Co3O4 NF and α-Fe2O3 HNF sensors to 50 ppm acetone gas, as a function of the operating temperature, were tested and the results are depicted in Fig. 5a. Obviously, the responses of each sensor are strongly dependent on the operating temperature. The responses of three sensors first increase with temperature, reach their maxima, and then decrease with a further increase in temperature. This decrease in responses at higher temperatures may be ascribed to the decrease in the number of active sites for acetone adsorption. The other possibility is that the rate of adsorption is lower than that of desorption at such high temperatures.23 Moreover, it is obvious that each sensor has an optimal operating temperature, at which the sensor exhibits the highest response to acetone. It is worth mentioning that the highest response of the Co3O4/α-Fe2O3 CSNF sensor to 50 ppm acetone is 11.7 (at 240 °C), which is almost 2.5 times higher than that of the Co3O4 NF and α-Fe2O3 HNF sensors. The results also demonstrate that the Co3O4/α-Fe2O3 core–shell nanostructures have an improved response in comparison to the single Co3O4 NFs and α-Fe2O3 HNFs. Thus, to obtain a deeper insight into the gas sensing behavior, attention is focused on the Co3O4/α-Fe2O3 CSNFs, which are the most efficient for acetone gas detection. 240 °C was chosen as the optimal operating temperature, at which the Co3O4/α-Fe2O3 CSNF sensor exhibited the highest response (11.7) for 50 ppm acetone.
Fig. 5b shows the responses of the Co3O4/α-Fe2O3 CSNF, Co3O4 NF and α-Fe2O3 HNF sensors to different concentrations of acetone at 240 °C. It can be easily seen that the responses of three sensors rapidly increase with increasing acetone concentration until 300 ppm. Above 300 ppm, the responses slowly change with the variations of acetone concentration, which indicate that the three sensors become more or less saturated. Furthermore, the sensor based on the Co3O4/α-Fe2O3 CSNFs exhibits higher responses to acetone at various concentrations compared with those based on the Co3O4 NFs and α-Fe2O3 HNFs.
For a highly effective gas sensor, the response time and recovery time are very important parameters for practical applications in addition to a superior sensitivity value. Fig. 6a shows typical response transients of the Co3O4/α-Fe2O3 CSNF sensor when it is exposed to 50 ppm acetone gas. The result indicates that the sensor immediately responds when acetone gas is introduced and then rapidly recovers to its initial value after the acetone is released. The response and recovery times were calculated to be 2 s and 20 s, respectively. The inset of Fig. 6a shows the dynamic response of the Co3O4/α-Fe2O3 CSNF sensor to different concentrations (1–50 ppm) of acetone gas at 240 °C. It is obvious that the response of the sensor changes rapidly on being exposed to acetone and to air, indicating the excellent reproducibility. In addition, the Co3O4/α-Fe2O3 CSNF sensor shows a clear and fast response change when exposed to a concentration of acetone as low as 1 ppm, with a response value of about 2.6.
Gas sensors for practical applications are required not only to possess high responses but also to present great selectivity to the target gases. Hence, a selectivity study of the Co3O4/α-Fe2O3 CSNF, Co3O4 NF and α-Fe2O3 HNF sensors was carried out by monitoring the sensors’ resistance changes toward 50 ppm of different gases at 240 °C. As shown in Fig. 6b, the Co3O4/α-Fe2O3 CSNF sensor shows an obviously high sensing response to acetone and is less sensitive to other gases, indicating that the Co3O4/α-Fe2O3 CSNF sensor has a very good selectivity for acetone gas. The excellent selectivity for acetone is mainly attributed to the enhanced reaction between the acetone and the absorbed oxygen at the optimal operating temperature.
The enhanced gas sensing performance of the Co3O4/α-Fe2O3 CSNF gas sensor should be ascribed to three aspects. On the one hand, the 1D structure of the nanofibers can easily transfer the gas molecules to and from the interaction region and improve the rate for charge carriers to transverse the barriers, which is induced by molecular recognition along the nanofibers.24,25 Moreover, the effective and rapid gas diffusion toward the surface regions of the nanofibers can be easily achieved by the large pores constituted by the space between nanofibers (as shown in Fig. 7a). Therefore, a short response time was obtained. The fast recovery behaviour can be explained as follows. When the sample is exposed to air again, the superficial layer of the Co3O4/α-Fe2O3 CSNF gas sensor contacts with oxygen molecules. Due to the existence of abundant electrons in the α-Fe2O3 shell, the oxygen easily captures the electrons from the sensing materials, which makes the resistance of the Co3O4/α-Fe2O3 CSNF gas sensor increase rapidly. Then, due to the rapid gas diffusion obtained by the large spaces between the nanofibers, more and more oxygen and sensing materials are involved in the adsorption process. In this way, the fast recovery is eventually achieved. On the other hand, the enhanced gas sensing performance is attributed to the extension of the electron depletion layer by the formation of a heterojunction between the p-type Co3O4 and the n-type α-Fe2O3.26,27 The heterojunction region is believed to easily attract reductive and oxidative gases (O2), thus forming a deeper electron depletion layer and causing a better sensing performance than pristine oxides.28 Specifically, Co3O4 and α-Fe2O3 are p-type and n-type semiconductor oxides, respectively. The heterojunction is formed at the interface between Co3O4 and α-Fe2O3 when they have an intimate contact between them (shown in Fig. 3a). Electrons will transfer from α-Fe2O3 to Co3O4 while holes will flow along the contrary direction of the electrons until the system obtains equalization of the Fermi levels. In this process, the built-in electric field in the interface of Co3O4 and α-Fe2O3 and the depletion layer can be formed (as shown in Fig. 7b). Due to the formation of a built-in electric field, the electrons and holes mainly flow in the α-Fe2O3 shell and the Co3O4 core, respectively. Moreover, Co3O4 is completely covered by α-Fe2O3 and the change of resistance mainly depends on the electron concentration and thickness of depletion layer. Therefore, the gas sensing performance of the Co3O4/α-Fe2O3 sensor results from the electron transport through the α-Fe2O3 shell. In addition, the depletion layer was formed in the interface of Co3O4 and α-Fe2O3, leading to the increase of resistance of the Co3O4 and α-Fe2O3 nanofibers. In other words, the main effect of the Co3O4 core (or the core–shell heterojunction) is that it decreases the carrier density in the α-Fe2O3 shell. That is to say, the Co3O4 core (or the core–shell heterojunction) increases the initial resistance of the Co3O4/α-Fe2O3 composite. When the electron concentration at the α-Fe2O3 shell becomes very low owing to the heterojunction, the injection of the same concentration of electrons by the gas sensing reaction leads to relatively higher variations in sensor resistance, which enhances the gas response. Accordingly, when the Co3O4/α-Fe2O3 sensor is exposed to acetone, the acetone molecules will react with the pre-adsorbed oxygen and release free electrons to the α-Fe2O3 shell, leading to an enhancement of the sensing response of the core–shell Co3O4/α-Fe2O3 sensor compared to the Co3O4 NF and α-Fe2O3 HNF sensors. Finally, according to the result of the elemental mapping of Fe, Co and O element of an individual Co3O4/α-Fe2O3 CSNF, a little Co is transferred to the α-Fe2O3 shell during the heating procedure. Thus, the well known Co3O4 catalytic activity will promote the reactions taking place during the sensing process (as shown in Fig. 7c). To evaluate the catalytic activity of Co, 1 wt% and 2 wt% Co-loaded α-Fe2O3 HNFs were prepared and the responses to different gases were tested. The results are shown in Fig. S1.† It can be observed that the loading of Co improves the selectivity of the samples. The 2 wt% Co-loaded α-Fe2O3 HNFs present a larger sensitivity enhancement to acetone in comparison with the α-Fe2O3 HNFs. However, for other gases, the responses of the 2 wt% Co-loaded α-Fe2O3 HNFs are slightly increased compared to the α-Fe2O3 HNFs.
A comparison between the sensing performances of the Co3O4/α-Fe2O3 CSNF based sensor and literature reports is summarized in Table 1. It can be easily seen that although the response value of the Co3O4/α-Fe2O3 CSNF sensor is not the highest, the optimal operating temperature (240 °C) is lower than some literature reports. Furthermore, the rapid response time (2 s) and recovery time (20 s) of the Co3O4/α-Fe2O3 CSNFs are also advantageous. Thus, taking the simple synthesis method and better acetone gas sensing performance into account, the Co3O4/α-Fe2O3 CSNFs can be developed to be highly effective acetone gas sensing sensors.
| Sample | Acetone (ppm) | S | tres/trec (s) | T (°C) | Ref. |
|---|---|---|---|---|---|
| In2O3 nanowire | 25 | ∼7 | —/— | 400 | 29 |
| Co-doped ZnO fiber | 100 | 16 | 6/4 | 360 | 30 |
| ZnO hollow sphere | 500 | ∼10 | <10/<10 | 300 | 31 |
| Cu doped WO3 hollow fiber | 20 | 6.4 | 5/20 | 300 | 32 |
| Y-doped SnO2 nanofibers | 50 | 12 | —/— | 300 | 33 |
| Nanoscale Fe2O3 structure | 100 | 15.7 | 0.8/27 | 240 | 34 |
| Co3O4/α-Fe2O3 CSNF | 50 | 11.7 | 2/20 | 240 | This work |
4. Conclusions
In summary, Co3O4/α-Fe2O3 core–shell nanostructures have been successfully fabricated through a facile coaxial electrospinning technique at an ambient temperature, followed by a subsequent thermal treatment. The sensor based on the as-prepared Co3O4/α-Fe2O3 core–shell nanostructures shows an excellent acetone gas sensing performance in terms of superior sensitivity, good selectivity, a low concentration detection limit, and rapid response and recovery times. The facile synthesis method and excellent gas sensing properties indicate that the as-prepared Co3O4/α-Fe2O3 core–shell nanostructures can be developed for acetone gas detection in the future.Acknowledgements
This research work was financially supported by the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT1017), the Graduate Innovation Fund of Jilin University (grant no. 20121108), the National Natural Science Foundation of China (grant no. 51102109), the Postdoctoral Science Foundation of China (grant no. 2012M510878), the Program for Science and Technology Development (no. 20110725) and the Postdoctoral Science Foundation of China (no. 2014T70289).Notes and references
- Y. Q. Liang, Z. D. Cui, S. L. Zhu, Z. Y. Li, X. J. Yang, Y. J. Chen and J. M. Ma, Nanoscale, 2013, 5, 10916 RSC.
- S. M. Wang, B. X. Xiao, T. Y. Yang, P. Wang, C. H. Xiao, Z. F. Li, R. Zhao and M. Z. Zhang, J. Mater. Chem. A, 2014, 2, 6598 CAS.
- A. Katoch, J. H. Byun, S. W. Choi and S. S. Kim, Sens. Actuators, B, 2014, 202, 38 CrossRef CAS PubMed.
- Y. V. Kaneti, Q. M. D. Zakaria, Z. J. Zhang, C. Y. Chen, J. Yue, M. S. Liu, X. C. Jiang and A. B. Yu, J. Mater. Chem. A, 2014, 2, 13283 CAS.
- X. Z. Wang, S. Qiu, C. Z. He, G. X. Lu, W. Liu and J. R. Liu, RSC Adv., 2013, 3, 19002 RSC.
- F. D. Qu, J. Liu, Y. Wang, S. P. Wen, Y. Chen, X. Li and S. P. Ruan, Sens. Actuators, B, 2014, 199, 346 CrossRef CAS PubMed.
- S. Park, H. Ko, S. Kim and C. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 9595 CAS.
- K. W. Liu, M. Sakurai and M. Aono, J. Mater. Chem., 2012, 22, 12882 RSC.
- J. Cao, Z. Y. Wang, R. Wang and T. Zhang, CrystEngComm, 2014, 16, 7731 RSC.
- Y. F. Li, Y. J. Hu, H. Jiang, X. Y. Hou and C. Z. Li, CrystEngComm, 2013, 15, 6715 RSC.
- F. D. Qu, Y. F. Wang, Y. Wang, J. R. Zhou and S. P. Ruan, RSC Adv., 2014, 4, 24211 RSC.
- D. L. Shao, H. T. Sun, G. Q. Xin, J. Lian and S. Sawyer, Appl. Surf. Sci., 2014, 314, 872 CrossRef CAS PubMed.
- J. N. Deng, L. L. Wang, Z. Lou and T. Zhang, RSC Adv., 2014, 4, 21115 RSC.
- Y. Y. Lu, W. W. Zhan, Y. He, Y. T. Wang, X. J. Kong, Q. Kuang, Z. X. Xie and L. S. Zheng, ACS Appl. Mater. Interfaces, 2014, 6, 4186 CAS.
- X. H. Rao, X. T. Su, C. Yang, J. D. Wang, X. P. Zhen and D. S. Ling, CrystEngComm, 2013, 15, 7250 RSC.
- P. Sun, Y. W. Liu, X. W. Li, Y. F. Sun, X. S. Liang, F. M. Liu and G. Y. Lu, RSC Adv., 2012, 2, 9824 RSC.
- D. Bekermann, A. Gasparotto, D. Barreca, C. Maccato, E. Comini, C. Sada, G. Sberveglieri, A. Devi and R. A. Fischer, ACS Appl. Mater. Interfaces, 2012, 4, 928 CAS.
- F. D. Qu, J. Liu, Y. Wang, S. P. Wen, Y. Chen, X. Li and S. P. Ruan, Sens. Actuators, B, 2014, 199, 346 CrossRef CAS PubMed.
- A. Katoch, S. W. Choi, G. J. Sun, H. W. Kim and S. S. Kim, Nanotechnology, 2014, 25, 175501 CrossRef PubMed.
- S. M. Wang, P. Wang, Z. F. Li, C. H. Xiao, B. X. Xiao, R. Zhao, T. Y. Yang and M. Z. Zhang, New J. Chem., 2014, 38, 4879 RSC.
- M. Righettoni, A. Tricoli and S. E. Pratsinis, Anal. Chem., 2010, 82, 3581 CrossRef CAS PubMed.
- J. Cao, H. M. Dou, H. Zhang, H. X. Mei, S. Liu, T. Fei, R. Wang, L. J. Wang and T. Zhang, Sens. Actuators, B, 2014, 198, 180 CrossRef CAS PubMed.
- X. M. Xu, X. Li, W. B. Wang, B. Wang, P. Sun, Y. F. Sun and G. Y. Lu, RSC Adv., 2014, 4, 4831 RSC.
- Z. J. Wang, Z. Y. Li, J. H. Sun, H. N. Zhang, W. Wang, W. Zheng and C. Wang, J. Phys. Chem. C, 2010, 114, 6100 CAS.
- J. Cao, T. Zhang, F. Li, H. Yang and S. Liu, New J. Chem., 2013, 37, 2031 RSC.
- C. W. Na, H. S. Woo, I. D. Kim and J. H. Lee, Chem. Commun., 2011, 47, 5148 RSC.
- Y. Q. Liang, Z. D. Cui, S. L. Zhu, Z. Y. Li, X. J. Yang, Y. J. Chen and J. M. Ma, Nanoscale, 2013, 5, 10916 RSC.
- Y. J. Liu, G. X. Zhu, J. Z. Chen, H. Xu, X. P. Shen and A. H. Yuan, Appl. Surf. Sci., 2013, 265, 379 CrossRef CAS PubMed.
- A. Vomiero, S. Bianchi, E. Comini, G. Faglia, M. Ferroni, N. Poli and G. Sberveglieri, Thin Solid Films, 2007, 515, 8356 CrossRef CAS PubMed.
- L. Liu, S. C. Li, J. Zhuang, L. Y. Wang, J. B. Zhang, H. Y. Li, Z. Liu, Y. Han, X. X. Jiang and P. Zhang, Sens. Actuators, B, 2011, 155, 782 CrossRef CAS PubMed.
- P. Song, Q. Wang and Z. X. Yang, Mater. Lett., 2012, 86, 168 CrossRef CAS PubMed.
- X. Bai, H. M. Ji, P. Gao, Y. Zhang and X. H. Sun, Sens. Actuators, B, 2014, 193, 100 CrossRef CAS PubMed.
- L. Cheng, S. Y. Ma, X. B. Li, J. Luo, W. Q. Li, F. M. Li, Y. Z. Mao, T. T. Wang and Y. F. Li, Sens. Actuators, B, 2014, 200, 181 CrossRef CAS PubMed.
- X. H. Sun, H. M. Ji, X. L. Li, S. Cai and C. M. Zheng, Sens. Actuators, B, 2014, 600, 111 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03675e |
| This journal is © The Royal Society of Chemistry 2015 |