Adsorption and sustained release of haemoglobin imprinted polysiloxane using a calcium alginate film as a matrix
Abstract
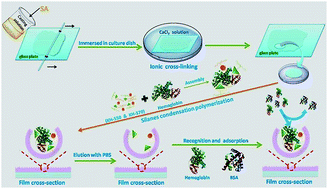
A calcium alginate (CA) hydrogel film was prepared by cross-linking sodium alginate (SA) with a CaCl2 solution. Haemoglobin (Hb) molecularly imprinted polysiloxane (MIP) was synthesized using silane as a functional monomer, Hb as a template and a CA hydrogel film as a matrix. SEM, TEM, TG and a water contact angle test were used to characterize the CA hydrogel and MIP film. The adsorption and recognition properties of MIPs were evaluated and the results showed that the MIP exhibited a significant improvement in terms of rebinding capacity for Hb compared with non-imprinted polysiloxane (NIP). Haemoglobin imprinted polysiloxane could selectively recognize the template protein from a mixture of other competitive proteins. The surface hydrophilicity and the swelling behaviour of the MIP film can be adjusted by changing the ratio of two types of silane. Hb was released from the MIP in a sustained manner in PBS and Tris–HCl solutions. The Higuchi model fits best for the MIP film no matter whether it is in PBS or in a Tris–HCl solution. It is a diffusion-controlled release mechanism for the MIP film. But diffusion is not the key factor for release of the NIP film.

 Please wait while we load your content...
Please wait while we load your content...