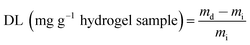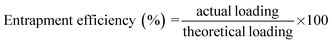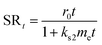Electroconductive smart polyacrylamide–polypyrrole (PAC–PPY) hydrogel: a device for controlled release of risperidone†
Suparna Sahaa,
Priyabrata Sarkar*a,
Mrinmoy Sarkarb and
Biplab Girib
aBiosensor Laboratory, Department of Polymer Science & Technology, University of Calcutta, 92 APC Road, Kolkata-700009, India. E-mail: sarkarpriya@gmail.com
bDepartment of Physiology, Experimental Medicine & Stem Cell Research Laboratory, West Bengal State University, Barasat, Kolkata-700126, India
First published on 10th March 2015
Abstract
Risperidone is a widely used antipsychotic drug for the treatment of schizophrenia and bipolar disorder. This paper discusses the preparation and use of a drug loaded electro-conductive hydrogel based on polyacrylamide and polypyrrole in the form of cylindrical devices to confer electro-actuable properties and also controlled release of the drug risperidone. The variation in the swelling and oxidation state of the material induced physical and chemical changes in the conducting polymer network. The electro-liberation process was optimized by tuning the properties of the composite. The hydrogel was characterized by various microscopic and spectroscopic analyses, swelling kinetics, and also network parameters. In vitro drug release studies were carried out in 0.1 N HCl and a phosphate buffer using dissolution apparatus with stirring. Cytotoxic results on HepG2 and C6 cells supported the biocompatibility of the hydrogel. A model was proposed for an implantable drug delivery device, where the dose could be adjusted by external signaling.
1. Introduction
In the human body, a neurotransmitter is present as an endogenous chemical that communicates information throughout the brain and body.1 Dopamine and serotonin are neurotransmitters known to be involved in regulating mood and behaviour. But over activity of dopamine is associated with a serious neurological disorder such as schizophrenia, a major therapeutic challenge of modern medicine and one of the last frontiers of brain research.2,3 The illness is defined by delusions, hallucinations, disorganized behaviour, and cognitive difficulties such as memory loss.4 There are several antipsychotic medications found to be effective for schizophrenic patients. The release of most of these drugs may be either improper or hindered and hence the patient is subjected to an overdose or side effects. Risperidone (RISP) is a well-established atypical antipsychotic water insoluble drug used for the treatment of mental disorders, like schizophrenia, bipolar disorder etc. by blocking the postsynaptic dopamine (D2) receptors.5 Patients with chronic mental health conditions are known to be poorly adherent to medication regimes. Controlled drug release systems offer advantages over conventional therapies by maintaining drug concentrations at therapeutically desired levels whilst simultaneously improving compliance.6 Risperidone is an ideal candidate for a controlled release system targeting mental health treatment.7 In polymer based controlled drug delivery systems (DDS), drug is incorporated into a polymeric network structure in such a way that the drug is released from the material in a predefined manner.8 Researchers have attempted to produce “smart” DDS by engineering the physical and chemical properties to specifically regulate permeability, environmental response, surface functionality, biodegradability, and biorecognition sites. It is essential to protect the drug from other molecules which could cause a change of chemical structure as well as pharmaceutical activity of drugs. Since the polymeric carrier must avoid interaction of the drug molecules with other molecules, it should be biodegradable, so that the chemical bonds that make up its chemical structure may break.9,10 Conducting polymers and hydrogels are macromolecular systems which present special characteristics that make them suitable for a wide range of practical applications in smart DDS. The electrical properties of conducting polymers make them suitable for devices driven by an external electrical signal, such as potential or current;11 on the other hand, hydrogels, which are tridimensional structures formed by the cross-linking of hydrophilic polymeric chains, present their swelling properties in solution depending on the chemical nature of the media, pH, ionic strength and temperature.12,13 The use of various natural hydrogels has already been reported e.g. chitosan, alginate, chitin, gelatin, pectin etc., but due to easy solubility of polysaccharides in water, these are not suitable to form stable hydrogels. Recently several synthetic hydrogels such as polyacrylamide, poly(acrylic acid), polyvinyl alcholol (PVA), polyvinyl pyrrolidone (PVP) have been used to improve the mechanical properties.14 Amongst all, polyacrylamide is a super porous hydrogel with superior biocompatibility, biodegradability, swelling and non-toxicity.15 The conducting polymers polypyrrole (PPY), polyaniline and poly (3,4-ethylenedioxythiophene) (PEDOT) have been used by many research groups as drug delivery devices.16,17 PPY based DDS offers additional promise due to its inherent conductivity, ease of preparation, apparent biocompatibility and more importantly release rate of drug can be altered on demand to match clinical requirements.18 This technology also opened up opportunities for implantable devices. For schizophrenia and related disorder, polymer implant is advancement over the present treatments based on antipsychotic medications. Some of these medications do manage symptoms but they are unable to treat the root of the problem. Successful implantation allows patients to manage their symptoms better, reduce their medications, and improve their quality of life.19Thus aim of the present work was to develop smart electro conductive hydrogel by electropolymerization of pyrrole inside the polyacrylamide hydrogel to impart multistimuli responsive properties. The research work also included optimization of hydrogel formulation, investigation of electrical signalling to modify the release rate of risperidone from hydrogel, kinetic study to explore the release mechanism and evaluation of toxicity of the synthesized smart hydrogel (PAC–PPY) to HepG2 and C6 cell in vitro for oral delivery as well as to be used as an implantable risperidone delivery device. A probable model for implantation was also presented for better understanding of its use for practical purpose.
2. Experimental
2.1 Reagent and chemicals
Acrylamide (AAM) was purchased from Himedia (Germany), recrystallized from acetone and oven dried before use. N,N′-Methylene-bis-acrylamide (MBA) and tetramethylethylenediamine (TEMED) were purchased from Merck (Germany) and were used as received for hydrogel preparation without further purification. Ammonium persulfate (NH4S2O4) and sodium nitrate (NaNO3) and p-toluene sulfonic acid (PTS) were procured from Merck (Germany). Pyrrole (Sigma) was purified by distillation prior use. Risperidone was purchased from M.P biomedicals (France) and used as received. HPLC grade acetonitrile and methanol were obtained from Merck (Germany).2.2 Synthesis of electro conductive hydrogel
The polyacrylamide hydrogel was prepared by free radical polymerization of acrylamide.20 Table 1 shows the compositions of four different formulations of polyacrylamide hydrogel with varying amount of the crosslinker. At first, fixed amount of acrylamide (AAM) and different quantities (0.1 wt%–0.6 wt%) of the crosslinker, N,N′-methylene-bis-acrylamide (MBA) were mixed in 10 mL of MillQ (Millipore, India) water. The solution was degassed for 10 minutes at room temperature. 20 μL of TEMED and 0.007 g of potassium peroxodisulfate were then added and mixed thoroughly. The pre-gel solution was injected in a fusion tube and gelation was completed after 10 minutes at room temperature. The hydrogel was placed in MilliQ water for extraction of impurities. The cylindrical shaped hydrogels of 0.5 cm diameter were used as substrates for the electrochemical synthesis of polypyrrole.21,22| Component | R1 | R2 | R3 | R4 |
|---|---|---|---|---|
| Acrylamide | 1.2 g | 1.2 g | 1.2 g | 1.2 g |
| MBA (wt%) | 0.6 | 0.3 | 0.15 | 0.1 |
| TEMED | 20 μL | 20 μL | 20 μL | 20 μL |
| Initiator | 0.007 g | 0.007 g | 0.007 g | 0.007 g |
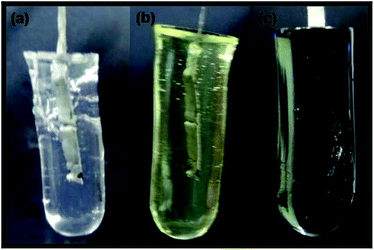
A thin platinum sheet was inserted in the hydrogel prepared and the assembly was used as working electrode (WE). Ag/AgCl and a platinum wire were used as reference and counter electrodes respectively. Before electropolymerization, the WE was immersed in electrolyte containing 0.1 M pyrrole, 0.1 M PTSA, 0.05 M risperidone, 1.0 mol L−1 sodium nitrate for 24 hours. Electropolymerization was carried out by cycling the potential from −0.35 V to 0.85 V at a scan rate of 30 mV s−1 for 30 cycles. After the synthesis, composite hydrogel (PAC–PPY) was placed in Mill Q water for 5 h in order to eliminate all soluble impurities. Fig. 1 depicts different stages of hydrogel formation and one may observe that black PPY permeated throughout the hydrogel on electropolymerization of pyrrole.
2.3 PAC–PPY hydrogel characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) potassium dihydrogen phosphate (pH 6.5; 0.05 M) (45
potassium dihydrogen phosphate (pH 6.5; 0.05 M) (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55, v/v) as the mobile phase. Isocratic flow was maintained at 1 mL min−1 with in-built UV for detection of risperidone at 237 nm. This method allowed for the concurrent quantification of both risperidone and PTSA concentrations on storage of hydrogel.
55, v/v) as the mobile phase. Isocratic flow was maintained at 1 mL min−1 with in-built UV for detection of risperidone at 237 nm. This method allowed for the concurrent quantification of both risperidone and PTSA concentrations on storage of hydrogel.2.4 Measurement of degree of swelling (Q) and swelling kinetic
Known amounts of hydrogels of different compositions were placed in water at room temperature. Periodically, hydrogels were carefully wiped with a tissue paper and weighed. The degree of swelling was calculated applying the following equation:| Q = (Wt − Wo)/Wo | (1) |
Swelling kinetics of the hydrogels were obtained by direct non-linear fitting of swelling data (SRt) of the hydrogels at different time intervals to the following second order rate equation23
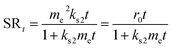 | (2) |
2.5 Analysis of hydrogel network parameter
Based on equilibrium swelling data, the number average molecular mass between crosslinks Mc, was calculated using Peppas–Merrill's theory. According to this theory number average molecular mass between the crosslink was calculated using following equation24
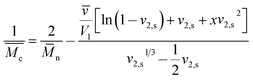 | (3) |
Again, crosslink density, q is defined as the mole fraction of crosslinked units:
| q = Mr/Mc, |
Another important parameter of the network is gel pore size ξ which can be calculated from the following equation26
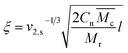 | (4) |
Crosslink density of hydrogel is obtained as,28
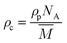 | (5) |
2.6 Study of drug loading and entrapment efficiency
Risperidone loading and entrapment efficiency in the hydrogel were carried out by a similar experiment reported elsewhere.29 The hydrogel samples of specified weight (mi) were first swollen in 50 mL PBS buffer of constant ionic strength (0.1 mol L−1) at pH 7.4 and containing specified amount of risperidone (mo). After 24 h of swelling, the drug loaded hydrogel were taken out from the buffer and washed with the same buffer to remove free drug from the surface. The remaining drug containing buffer solution was analyzed by UV-spectrophotometer at the λmax 237 nm.Thus drug loading (DL) and entrapment efficiency of the hydrogel was determined as
where md is weight of drug loaded dry hydrogel sample.
2.7 In vitro release study
The release of risperidone from PAC–PPY hydrogel was monitored as a function of incubation time in PBS at 37 °C using dissolution apparatus stirred at 50 rpm. The samples were immersed in 50 mL of PBS (pH 7.4, 1.2, 6.8). Specific volumes were withdrawn for measurement of risperidone and same volumes were replaced with similar dissolution media to maintain sink condition. The concentration of risperidone was determined by a UV-vis Spectrophotometer (CECIL CE7200, 7000 series, UK) by observing optical density (OD) at 237 nm. The drug release data were fitted to a number of kinetic models e.g. zero-order, first-order, Higuchi's square root of time, and Korsemeyer–Peppas's equation30–33 to determine the release rate (K) and coefficient of determination (R2).2.8 Electrical stimulation of drug-loaded PAC–PPY hydrogel
The release of RISP from PAC–PPY was triggered using cyclic and differential pulse voltammograms (CV, DPV) where polypyrrole encapsulated polyacrylamide hydrogel was used as working electrode. The voltage was swept from −0.8 to +1.4 V with a scan rate of 100 mV s−1. The amount of RISP released was quantified as described in Section 2.7.2.9 In vitro cytotoxicity test via Alamar Blue assay
The C6 glioma cell line was obtained from American Type Culture Collection (Rockville, MA, USA). Maintaining the standard protocol (for 96-well assay), cell proliferation was assessed using Alamar Blue assay technic (THE CELLTITER-BLUE™ CELL VIABILITY ASSAY; Promega Corporation, Madison, USA). 100 microliters of glioma cell suspension (C6) in MEM-α and supplemented with 10% fetal bovine serum containing 1 × 104 cells were added to each well of a 96-well microtiter plate separately. C6 cells were tested in presence and absence of different doses of RISP and PAC–PPY hydrogel. Plates were incubated at standard cell culture conditions for 24 h. Twenty microliters of Celltiter-Blue™ reagent was then added in each well and allowed to incubate at 37 °C in 5% CO2 for 3 h. The percent reduction of the dye reagent was determined measuring the absorbance at 570 nm with 595 nm wavelength used as a reference wavelength using a microplate absorbance reader (BIO-RAD, California 94547, USA). From the observed values of absorbance, the percent viability of the cells was calculated.343. Results and discussions
3.1 Study of swelling kinetics
The swelling ratio of the hydrogel depends on the chemical structure of the polymer and this is directly related to loading capacity of drug into the polymer and also release rate of drug from it. Swelling studies of composite hydrogels were carried out at different pH (1.2, 6.8, and 7.4) at room temperature (25 °C) (Fig. 2a). It was observed that swelling ability of the hydrogel did not change significantly in presence of PPY and this was also supported by the previous findings.35 Optimum swelling was observed at pH 7.4 whereas, a lesser % swelling (Ps) was observed in acidic as well as alkaline medium. In presence of acid and base, the amide groups of polyacrylamide could be converted into carboxylate ion (COO−) due to hydrolysis and resulted in the repulsion from COO− ions on the backbone molecules. In acidic medium, COO– COO− ion repulsion was screened by H+ ions which did not allow the network to expand resulting in decreased Ps. Further increase in acidic character resulted in increased concentration of H+ ions enabling increased screening effect and decreased COO– COO− ion repulsion and hence a decreased Ps. Similarly, in case of basic medium –COO− –COO− repulsive forces were screened by Na+ ion causing less Ps. However at pH 7.4, water molecules underwent hydrogen bonding with amide group forming a cage-like structure to allow accommodation of more and more water molecules for the swelling of polyacrylamide matrix.36 Again, the chemical structure was depended on composition of the polymeric matrix e.g. the crosslinking ratio. The higher the crosslinking ratio the tighter was the hydrogel and hence swelled less compared to the same hydrogels with lower crosslinking ratios. Crosslinking hinders the mobility of the polymer chain lowering the swelling ratio. From Fig. 2a it could be depicted that highest % swelling was obtained for R3, and the order of % swelling was R3 > R2 > R1 > R4. But stability of different hydrogel was in the order R1 > R2 > R3 > R4. The crosslinking densities of R3 and R4 were too low to maintain the porous integrity for a long time.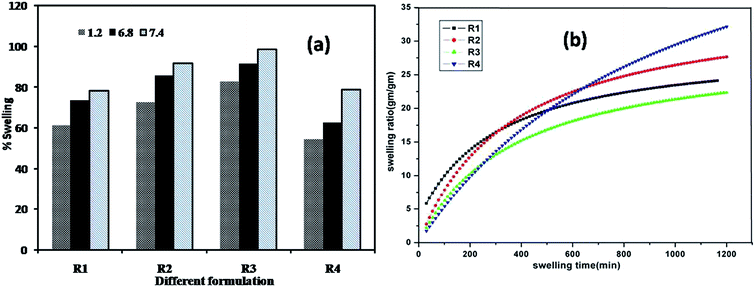 | ||
| Fig. 2 (a) Swelling study of different formulations at different pH (b) non-linear fitting of swelling data to second order rate equation. | ||
The swelling ratio (SR) of the conductive hydrogels at definite time intervals were analyzed to fit to the non-linear second order rate equation
The data fitting were carried out by Levenberg–Marquardt (L–M) algorithm using Origin 8.5 software to determine the rate constant (ks2) and initial rate of swelling (r0) by iteration using chi square (χ2) and F values. The trend line of different formulation of hydrogel was shown in Fig. 2b. The values of ks2 and ro, experimental and calculated ESR (equilibrium swelling ratio) and other statistical parameter of the hydrogels are given in Table S1 (ESI†). It was observed that the regression coefficient R2 of all these formulations were close to unity (0.96–0.98) while chi square (χ2) of all of them low (0.72–0.94) and F values were very high except formulation R4. Further, calculated ESR of the hydrogels was also observed to be very close to its experimental ESR. This fact confirmed that swelling data could very well fit to the second order rate equation. The decrease in the swelling rate with increase in crosslinker concentration suggested that specific interaction between polymer network and medium were weak.
3.2 Hydrogel network parameters and drug loading capacity
The important parameters that describe the network of hydrogel are the average molecular weight of the polymer chains between the two consecutive crosslinks (Mc) and the corresponding mesh size (ξ). These network parameters and also crosslink density (ρc) were calculated using eqn (3)–(5) based on swelling data of these hydrogels in Milli Q water at pH 7.4. Due to the random nature of the polymerization process, only average values of Mc could be calculated. The mesh size, or the correlation distance between two adjacent crosslinks provides a measure of the space available between the macromolecular chains available for diffusion and movement of drug molecule. As the gel swells, the cross linked chains widen, thus increasing the mesh size and allowing solute transfer from the gel. As shown in Table S1 (ESI†), the values of Mc and ξ decreased while ρc increased with the increasing amount of crosslinker indicating that network meshes denser with the higher quantity of crosslinker. This was due to increased number of networks in the gel matrix with increase in crosslinker concentration.It was observed that loading and entrapment efficiency increased with decrease in crosslinker amount whereas increase in crosslink density reduced the swelling of hydrogel and thus less amount of drug was accommodated inside the hydrogel. The drug entrapment capacity was found to be more than 70% in all formulation indicating this smart hydrogel performed as a good reservoir device for drug. It was also investigated that the amount of the loaded drug in hydrogel was significantly affected by the impregnation time. With increasing loading time, the amount of drug loaded initially increased and then began to stabilize. The maximum loading of drug was achieved for a 5 h loading time.
3.3 Surface morphology of polymer hydrogel
The surface morphologies of the hydrogel and drug loaded hydrogel were observed by SEM analysis. Fig. 3a shows pristine hydrogel with integrated pore structure and interconnected channels that could easily encapsulate the drug molecule. After loading of drug (Fig. 3b), integrity of pore structure remained intact and this was essential for further electro-polymerization of pyrrole inside the hydrogel. SEM study further revealed formation of polypyrrole inside the hydrogel but some pores were still open to allow swelling (Fig. 3c). It might be noticed (Fig. 3d) that the interconnected network was totally lost due to erosion of polymer chain after the release of drug.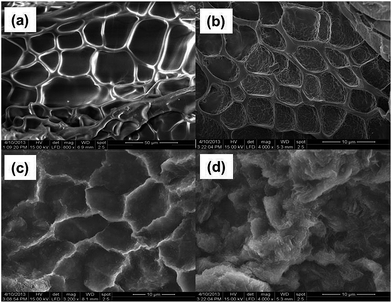 | ||
| Fig. 3 SEM images of freeze-dried (a) pristine PAC hydrogel (b) RISP loaded PAC hydrogel (c) PAC–PPY–RISP hydrogel (d) after RISP release. | ||
From the SEM image of RISP loaded PAC–PPY hydrogel, drug molecule was not clearly observed. Thus FESEM was performed to confirm drug encapsulation in polymer hydrogel. It could be seen from Fig. 4a that spherical RISP molecules were entrapped inside the hydrogel. The presence of F in EDAX result corroborated the FESEM result shown in Fig. 4b.
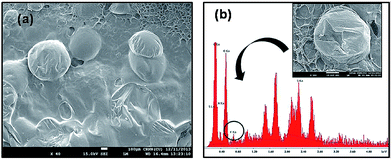 | ||
| Fig. 4 FESEM images of drug loaded PAC–PPY hydrogel (a) and EDAX spectra of PAC–PPY, RISP molecule (inset). | ||
3.4 Raman spectroscopy
The molecular structure of the prepared hydrogel was analyzed by Raman Spectroscopy and the results were shown in ESI (Fig. S1†).3.5 Risperidone release study
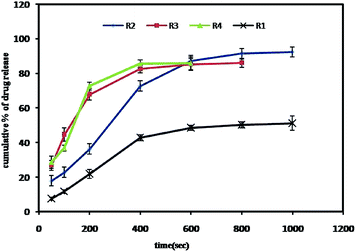
Drug release kinetics were conducted in 900 mL of 0.1 N HCl (pH 1.2) and 900 mL of phosphate buffer (pH 6.8 and pH 7.4) kept at thermostatically controlled temperatures of 37 ± 0.5 °C using a stirred apparatus run at 100 rpm for different formulations. Data obtained from in vitro release studies were fitted to various kinetic models to elucidate the drug release mechanism (shown in ESI, Table S2†) and also a release profile (Fig. 6) was studied to observe the concentration change over time with application of electric field.It could be seen that R2 and R3 exhibited higher cumulative as well as sustained drug release compared to R1 (highest crosslinking density) and R4. The formulations R3 and R4 with lower cross linking density released most of the drug within 400 s under the application of electric field (−0.4 V) and as a result there was no significant release after 600 s due to their unstable nature. R2, on the other hand, exhibited prolonged release pattern up to 1000 s with cumulative drug release 92%. R1 with highest cross link density could achieve only about 45% even after 1000 s. Though R2 resulted in initial slow release compared to R3 and R4, with the prolonged release pattern should be helpful as an implantable device to achieve the therapeutic plasma concentration of risperidone for schizophrenic patient.37
3.6 Electrochemical controlled release
For neurological disorder it is very important to investigate the effect of electrical signalling that can be used to control and modify the release of risperidone from conductive hydrogel by altering the redox states. The liberated RISP was measured by UV absorbance at 237 nm. Polypyrrole is known to have switching properties and this was evident by the appearance of characteristic anodic and cathodic peaks as seen in Fig. 7 (inset). This redox switching was accompanied by swelling and shrinking of the polymer material due to the incorporation of hydrated ions from surrounding or release of drug molecule from the polymer. To ascertain the effect of potential on risperidone release, voltammetric study was performed between −0.6 to 0.6 V. This potential window was selected because it included the crossover point from cathodic to anodic currents. It is known that RISP release occurs through diffusion and structural relaxation of PAC–PPY structure. In our experiment different potential of −0.6, −0.4, −0.2, 0.2, 0.4, 0.6 were applied and each potential was maintained for 100 s. Risperidone release was observed to be maximum at −0.4 V as PTSA doped PAC–PPY hydrogel swelled maximum on reduction. As per the ESCR model, the polymer begins to swell at the potential where the oxidation starts during a voltammetric experiment, and shrinks at the beginning of the reduction.38 However, in the present study, the fact was reverse due to the dopant PTSA present in the polypyrrole. There could be various possible reasons for volume changes. One was phase relaxation in PPy film which involved solvent transport through the polymer and slow structural rearrangement of the PPy chains. Another was ion transport for charge compensation inside the PPy film i.e. dopant anion export or cation import from or to the PPy respectively during the reduction process.39 Since p-toluenesulfonate is a big ion and its hydrophobic interaction with the PPy matrix is strong, the reduction of PPy was most likely associated with cation insertion from the solution, which expanded the PPy and caused a volume increase. Similar phenomenon has been reported for PPy doped with other big organic anions.40 Since the reduction potential of PAC–PPY hydrogel has been observed at −0.35 V, large amount of drug release occurred due to maximum swelling on application of −0.4 V. The subsequent slow reduction in volume might be associated to the structural relaxation of PPy film.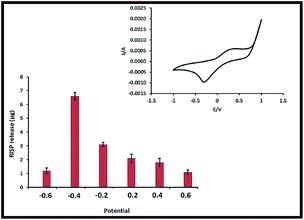 | ||
| Fig. 7 Effect of different potential on RISP release (inset: cyclic voltammetry of RISP loaded PAC–PPY). | ||
Significantly at the potential window i.e. 0.2 to 0.6 V, less RISP was released when the hydrogel was held in the oxidized state, despite the repulsion forces the cationic risperidone would experience from the positive charge of the polymer backbone. The lower amount of drug release was attributed to the more compact nature of the polymer which decreased the permeability of risperidone through the film. Thus risperidone release was depended on the structural conformation of polymer matrix rather than charge of the polymer backbone. This behaviour clearly indicated that encapsulation of RISP in PAC–PPY polymer prevented the undesired release from the hydrogel and it could be modulated on the application of electric field.
3.7 Stability study by HPLC
HPLC is a predominant tool used to analyze the drug substance and the impurities, particularly for small molecules. The excipients used in this formulation were pyrrole and PTS. For the chromatographic method to be applicable to drug release studies, it should resolve the risperidone peak from exicipent peaks and any other degradation products formed during preparation. The peaks at 2.5, 2.7, 4.6 and 5.3 min were identified as PBS, PTS, risperidone and pyrrole respectively (Fig. 8a).41,42 In addition, the method should be capable of quantifying risperidone in the presence of any excipients that could be co-released with the drug. HPLC analysis of released samples from freshly prepared PAC–PPY hydrogel showed the presence of unreacted pyrrole monomer after 1 h. However, no pyrrole was detected in release samples of PAC–PPY hydrogel aged for 20 days. This might indicate polymerization of monomer on storage and this should have contributed to the decrease in pyrrole amount observed after 6 days of storage at 40 °C (Fig. 8b). However, no pyrrole or any other impurity was detected in release samples from PAC–PPY even after 20 days storage (Fig. 8c).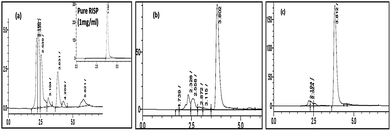 | ||
| Fig. 8 HPLC chromatograms of RISP release from PAC–PPY hydrogel (a) after 1 h (b) 6 days and (c) 20 days. | ||
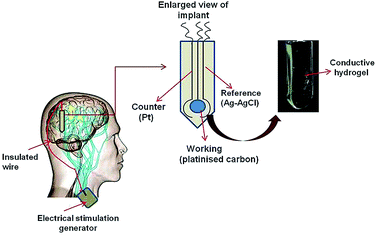
3.8 Plausible model of implantation and evaluation of cell viability
Though our present system consisted of three different electrodes, the implantable system should be a compact assembly of miniaturized electrodes. This composite electrode could be used as micro device, thin needle shaped with insulated wire (shown in Fig. 9). It should be inserted through small opening of the skull and then implanted in the brain. The extension of this would be an insulated wire passing under the skin of the head, neck, and shoulder, connecting the lead to the implantable pulse generator. The IPG (the “battery pack”) would be the third component implanted under the skin near the collarbone.43An important prerequisite for the polymer implant system is that it should not provoke toxic effects on healthy cells and tissues. As this polymeric device would be implanted surgically in the frontal lobe of the brain to regulate the stimulation of neurons to alleviate symptoms from schizophrenia and related disorders so viability and stability of surrounding brain tissue/cell should be studied. It has been postulated that targeting the synthesis and secretion of neurotrophic factors, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF), might be a new approach for treating neurodegenerative and depressive disorders. GDNF release from glial cell, a trophic factor for dopaminergic and other neurons which protect them from degeneration associated with diseases such as schizophrenia or Alzheimer's.44 According to the literature, there is a decrease in glial cell number or hypofunction of glial cells in depression. It was also found that both antidepressants and atypical antipsychotics might target glial cells, and that they increase the release of glial-cell-line-derived neurotrophic factor (GDNF). As the most abundant glial cell type in the brain, astrocytes have roles in modulation of synaptic transmission and uptake of neurotransmitter, regulation of extracellular ion concentrations and metabolic support of neuron. Hence C6 glioma cell, a rich source of GDNF45 was used as model cell for the in vitro cytotoxicity study.
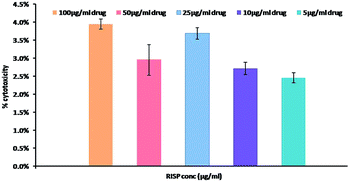
To observe the cytotoxicity, C6 glioma cells were incubated for 24 h with different concentration of risperidone as well as PPY–PAC with or without risperidone encapsulation. It was already established that optimized formulation for risperidone release was R2. Hence R2 was chosen for cell viability test of various concentrations. Using Alamar-Blue assay, it was observed that risperidone had no significant effect on cell death. The percentages of dead cells are only 4% even after 100 μg mL−1 risperidone treatment for 24 h (shown in Fig. 10).
Further, the data obtained from Alamar-Blue assay after 24 h incubation is shown in Fig. 11a and b. It could be seen that both PAC–PPY and RISP loaded PAC–PPY treatment did not produce much effect on cell growth or cell density and 90–92% cells were viable even up to 1000 μg mL−1 polymer hydrogel and 50 μg mL−1 risperidone. Thus it could be said that C6 cell viability was not significantly affected on dose of polymer hydrogel up to 1000 μg mL−1 with a drug up to 50 μg mL−1. In culture condition, C6 cells exhibited fibroblast-like morphology (Fig. 11c). However, in the presence of high doses of risperidone, cells might be stellate, with process-bearing cells and partial retraction of the cell body, but our model of investigation during 24 h incubation showed risperidone to be able to interact with C6 cells without prominent alterations on cell integrity or viability. Moreover, no noticeable changes in morphology of the C6 cells were observed in comparison to the control cells.
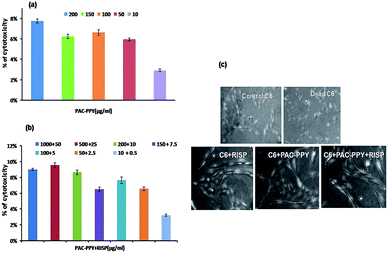 | ||
| Fig. 11 Effect of PAC–PPY (a) RISP loaded PAC–PPY (b) on C6 cell line measured from Alamar-Blue assay and phase contrast optical micrographs of C6 cell line (c) after 24 h incubation. | ||
Again, after treatment with pristine hydrogel (10–200 μg mL−1) and risperidone loaded hydrogel particles of various concentrations (10–1000 μg mL−1) no significant change of cell morphology was observed compared with the control. In presence of hydrogen peroxide (H2O2) [i.e. positive control], most of the cells however, underwent apoptotic/necrotic cell death. Thus cells had lost their typical fibroblast like morphology of finger like projections and membrane integrity. Prominent membrane blebbing, vacuolar appearance in the cytoplasm, fragmented cellular and nuclear integrity, cell shrinkage and typical necrosis or necroptosis like cellular morphology were clearly observed in C6 glioma dead cells. But most of the cells maintained fibroblast morphology even after treatment with 200 μg mL−1 pristine hydrogel and 10 μg mL−1 RISP loaded hydrogel.
In case of oral delivery, risperidone loaded hydrogel might be metabolised by a process in which risperidone or hydrogel could be chemically altered by cells to more water soluble metabolites to allow elimination through urine or bile or by increasing access to drug excretory transporters. The liver is perfectly designed as a drug removal organ. Hence the HepG2 cell line was also used for evaluating the toxicity of chemicals and drugs and data obtained from MTT assay and shown in ESI.†
4. Conclusions
A smart electroactive hydrogel was developed by electropolymerization of pyrrole inside the pores of polyacrylamide hydrogel. This allowed the hydrogel to be used as a device for controlling electrochemical phenomena for the liberation of RISP molecules. The extended release of risperidone from hydrogel matrix was investigated for 24 hours in vitro study. The release profile obtained from kinetics study showed that formulation R4 could achieve more prominent linear release compared to other compositions. The specific release of RISP with time and stability of the composite hydrogel were satisfactory. Electrical stimulus could alter the redox state of PAC–PPY hydrogel and modify the release profile of risperidone. The rate of RISP release could also be controlled through electrical stimulation. Thus this smart hydrogel could improve therapeutic activity and reduce chances of the dose-dependent side effects most often associated with conventional tablets for treatment of chronic mental health condition. Cytotoxicity PAC–PPY was investigated with HepG2 cell line and also in presence of C6 cell line and the results confirmed the material to be nontoxic and biocompatible with both. Thus the novel RISP loaded conductive hydrogel could be useful for the development of the surgically implantable devices as well as oral delivery.Acknowledgements
The author (SS) is indebted to DST (INSPIRE), New Delhi for her fellowship. The authors acknowledge JNU, New Delhi and CRNN, TEQIP of University of Calcutta for providing assistance in Raman and SEM analysis.Notes and references
- L. J. Elias and D. M. Saucier, Neuropsychology: Clinical and Experimental Foundations, Pearson, Boston, 2005 Search PubMed.
- A. M. McIntosh, S. M. Maniega, G. K. Lymer, J. McKirdy, J. Hall, J. E. Sussman, M. E. Bastin and J. D. Clayden, Biol. Psychiatry, 2008, 64, 1088 CrossRef PubMed.
- K. L. Davis, R. S. Kahn, G. Ko and M. Davidson, Am. J. Psychiatry, 1991, 148, 1474 CrossRef CAS PubMed.
- C. Nancy, M. D. Andreasen and M. Flaum, Schizophr. Bull., 1991, 17, 27 CrossRef.
- P. A. J. Janssen, C. J. E. Niemegreers, F. Awouters, K. H. L. Schellekens, A. A. H. P. Megens and T. F. Meert, J. Pharmacol. Exp. Ther., 1987, 244, 685 Search PubMed.
- J. A. Lieberman, T. S. Stroup, J. P. McEvoy, M. S. Swartz, R. A. Rosenheck, D. O. Perkins, R. S. Keefe, S. M. Davis, C. E. Davis, B. D. Lebowitz, J. Severe and J. K. Hsiao, N. Engl. J. Med., 2005, 353, 1209 CrossRef CAS PubMed.
- P. Chue, R. Welch and C. Binder, Can. J. Psychiatry, 2004, 49, 701 Search PubMed.
- E. K. Uhrich, Chem. Rev., 1999, 99, 3181 CrossRef PubMed.
- V. Jogani, K. Jinturkar, T. Vyas and A. Misra, Recent Pat. Drug Delivery Formulation, 2008, 2, 25 CrossRef CAS.
- W. R. Gombotz and D. K. Pettit, Bioconjugate Chem., 1995, 6, 332 CrossRef CAS.
- P. M. George, D. A. LaVan, J. A. Burdick, C. Y. Chen, E. Liang and R. Langer, Adv. Mater., 2006, 18, 577 CrossRef CAS.
- Y. Hu, K. Horie, T. Tori, H. Ushiki and X. Tang, J. Polym., 1993, 25, 123 CAS.
- H. Sasase, T. Aoki, H. Katono, K. Sanui and N. Ogata, Makromol. Chem., Rapid Commun., 1992, 13, 577 CrossRef CAS.
- N. A. Peppas, P. Bures, W. Leobandung and H. Ichikaw, Eur. J. Pharm. Biopharm., 2000, 50, 27 CrossRef CAS.
- D. Torresi, S. I. C. Lira and L. Marcos, Electrochem. Commun., 2005, 7, 717 CrossRef PubMed.
- B. L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481 CrossRef.
- J. Che, Y. Xiao, X. Zhu and X. Sun, Polym. Int., 2008, 57, 750 CrossRef CAS.
- P. M. George, A. W. Lyckman, D. A. LaVan, A. Hegde, Y. Leung and R. Avasare, Biomaterials, 2005, 26, 3511 CrossRef CAS PubMed.
- J. Siegel Steven, I. Winey Karen, E. Gur Raquel, H. Lenox Robert, B. Bilker Warren, D. Ikeda, N. Gandhi and W.-X. Zhang, Neuropsychopharmacology, 2002, 26, 817 CrossRef CAS.
- D. S. G. Hu and M. T. S. Lin, Polymer, 1994, 35, 4416 CrossRef CAS.
- C. J. Small, C. O. Too and G. G. Wallace, Polym. Gels Networks, 1997, 5, 251 CrossRef CAS.
- C. Barthus Rosângela, M. Luiz Lira and I. S. I. Córdoba de Torresi, J. Braz. Chem. Soc., 2008, 19, 630 CrossRef PubMed.
- I. D. Mall, V. C. Srivastava, G. V. A. Kumar and I. M. Mishra, Colloids Surf., A, 2006, 278, 175 CrossRef CAS PubMed.
- S. Lu and K. S. Anseth, Macromolecules, 2000, 33, 2509 CrossRef CAS.
- M. Sen, A. Yakar and O. Guven, Polymer, 1999, 40, 2969 CrossRef CAS.
- T. Canal and N. A. Peppas, J. Biomed. Mater. Res., 1989, 23, 1183 CrossRef CAS PubMed.
- J. Brandrup and E. H. Immergut, Polymer Handbook, John Wiley and Sons, New York, 3rd edn, 1989, p. 53 Search PubMed.
- K. B. Sunil and S. Surinderpal, React. Funct. Polym., 2006, 66, 431 CrossRef PubMed.
- S. Hua, H. Ma, X. Li, H. Yang and A. Wang, Int. J. Biol. Macromol., 2010, 46, 517 CrossRef CAS PubMed.
- Release Kinetics, Data Interpretation, in Encyclopedia of Controlled Drug Delivery, ed. B. Narashimhan, S. K. Mallapragada and N. A. Peppas, John Wiley and Sons, Inc, New York, 1999, p. 921 Search PubMed.
- D. W. Bourne, Pharmacokinetics, in Modern pharmaceutics, ed. G. S. Banker and C. T. Rhodes, Marcel Dekker Inc, New York, 4th edn, 2002 Search PubMed.
- M. Grassi and G. Grassi, Curr. Drug Delivery, 2005, 2, 97 CrossRef CAS.
- N. A. Peppas, Eur. J. Pharm. Biopharm., 2000, 50, 27 CrossRef CAS.
- T. J. Mossman, J. Immunol. Methods, 1983, 65, 55 CrossRef.
- B. C. Kin, G. M. Spinks, G. G. Wallace and R. John, Polymer, 2000, 41, 1783 CrossRef.
- A. Thakur, R. K. Wanchoo and P. Singh, Chem. Biochem. Eng. Q., 2011, 25, 181 CAS.
- A. Navitha, S. Jogala, C. Krishnamohan and J. Aukunuru, J. Adv. Pharm. Technol. Res., 2014, 5, 84 CrossRef PubMed.
- I. J. Suárez, T. F. Otero and M. Marquez, J. Phys. Chem. B, 2005, 109, 1723 CrossRef PubMed.
- S. H. Takahashi, L. M. Lira and S. I. Córdoba de Torresi, J. Biomater. Nanobiotechnol., 2012, 3, 262 CrossRef CAS.
- S. I. Córdoba de Torresi and L. M. Lira, Sens. Actuators, B, 2008, 130, 638 CrossRef PubMed.
- S. Darren, T. Sejdic Jadranka and G. Sanjay, J. Chromatogr. Sci., 2011, 49, 780 Search PubMed.
- S. Darren, E. Bryon, J. Wright, S. Travas, R. Anthony and G. Sanjay, Sens. Actuators, B, 2010, 151, 97 CrossRef PubMed.
- F. M. Weaver, K. Follett and M. Stern, J. Am. Med. Assoc., 2009, 301, 63 CrossRef CAS PubMed.
- Y. Obara and N. Nakahata, Drug News Perspect., 2002, 15, 290 CAS.
- K. Hisaoka, A. Nishida and T. Koda, J. Neurochem., 2001, 79, 25 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Raman spectroscopy, risperidone release kinetic and cytotoxicity of HepG2 cell. See DOI: 10.1039/c5ra03535j |
| This journal is © The Royal Society of Chemistry 2015 |