Adsorption enhancement of methylene blue dye at kaolinite clay–water interface influenced by electrolyte solutions†
Khushi Mukherjee,
Ankit Kedia,
K. Jagajjanani Rao,
Satarupa Dhir and
Santanu Paria*
Interfaces and Nanomaterials Laboratory, Department of Chemical Engineering, National Institute of Technology, Rourkela 769008, Orissa, India. E-mail: santanuparia@yahoo.com; sparia@nitrkl.ac.in; Fax: +91 661 246 2999
First published on 26th March 2015
Abstract
The contamination of surface water by dyes released from the effluent of textile industries is a major environmental concern. Adsorption is a cheap and easy separation technique to remove dyes from the effluent water. In this study, the adsorption behaviors of a widely used cationic dye, methylene blue (MB), on kaolinite clay surface in the presence and absence of electrolytes have been reported. The adsorption isotherms of MB in the absence of electrolytes follow the Langmuir model, however, in the presence of electrolytes they follow the Freundlich model. At a constant dye concentration (below the saturation equilibrium concentration), the dye adsorption increases linearly along with the increasing ionic strength of the electrolyte solutions. Among the four electrolytes (NaCl, CaCl2, Na2SO4, Na2HPO4) studied here, Na2HPO4 has the highest adsorption enhancement ability, ∼127% with respect to that of pure MB at a 2 mM initial concentration. This study shows that the adsorption capacity of kaolinite clay can be enhanced significantly by the use of electrolytes, which is very useful for the remediation of dye contaminated waste water.
1. Introduction
Effluents from different industries such as textile, leather, paper, and printing are highly contaminated with dyes, and are becoming a major concern to the environment due to their potential toxic effects to humans and aquatic life.1,2 Many traditional techniques for controlling environmental pollution such as flocculation, chemical oxidation, and membrane separation have proved to be less effective processes for water soluble dyes. However, adsorption is considered to be a reliable and potential technique for removal of dyes from contaminated effluents.3–5 The adsorption of dyes has been performed using several adsorbents such as activated charcoal,6,7 polymeric resins,8 sugarcane bagasse,9 biological molecules,10 and so on, which make the process economic, eco-friendly, and simple.Because of the anionic nature of the clay particles in aqueous media, a number of studies have been reported using clay mineral for removing dye from waste water.11 Since clays are available in nature abundantly, clay based adsorption processes are in general economic, to treat a huge amount of industrial effluents. Among different clays, kaolinite is a widely used clay for different applications for its easy availability and inexpensive. Kaolinite clay structurally consists of two layers, tetrahedral Si layer and octahedral Al layer which are interlinked.12 The charged layers are neutralized by the presence of Na and K ions and the interlayer space also contain some amount of water.12,13 The water present in the interlayer space of the kaolinite molecule can be removed by annealing the clay at high temperature, which may increase the dye adsorption capacity13 but the processing cost will increase significantly.
Methylene blue adsorption from aqueous media has been reported using different clay adsorbents such as montmorillonite, nontronite,14 laponite,15 sepiolite,16 Egyptian smectitic,11 montmorillonite and nontronite14 etc. The change in rheological16 and other properties of clay after adsorption of dye was also studied. The adsorption of methylene blue is also used to calculate the percentage of bentonite mud present in the drilling circulation fluid, determination of cation exchange capacity, and surface area of clay.17,18 The effects of different parameters on dye adsorption capacity such as pH, temperature, adsorbent dose, cation exchange capacity, etc. were reported in the literature.19,20 In a recent study it has been reported that, the acid treatment (HCl) of bentonite clay significantly (∼95%) enhances the adsorption capacity of Reactive Red 223 dye onto bentonite clay.21 In recent years some researchers including our group have reported that the addition of electrolytes significantly enhances the adsorption capacity of surfactants on different solid surfaces, which in turn leads to decrease the consumption of surfactants.22–27
In the present study, we report the effect of electrolytes of different valances on the methylene blue adsorption onto the kaolinite clay–water interface. The adsorption isotherms in the presence and absence of electrolytes have been fitted with the Langmuir and Freundlich isotherm models to know the adsorption behavior. Different characterization techniques such as zeta potential, FT-IR, and XRD were also used to explore the mechanism of adsorption in the presence of electrolytes. As the electrolytes are cheap and nontoxic to the environment, the adsorption capacity enhancement of the clay as adsorbent in the presence of the electrolytes may be effectively useful for the remediation of dye contaminated industrial waste water. To the best of our knowledge, the adsorption enhancement of dye onto the clay–water interface in the presence of different electrolytes has not been reported before.
2. Materials and methods
2.1 Materials
Kaolinite clay was purchased from Loba Chemie and analytical grade MB, NaCl, Na2SO4, CaCl2, and Na2HPO4 were purchased from Merck.2.2 Methods
3. Results and discussion
3.1 Adsorption isotherm
In the adsorption study, adsorption kinetics was investigated and the equilibrium time found to be 10 min that indicates a rapid adsorption process. Since the process is fast, for the isotherm study, we used 30 min equilibrium time for all experiments. The Fig. 1 represents the adsorption isotherm of MB on kaolinite clay surface. The figure depicts a sharp increase in the amount of adsorbed dye at lower concentration, but at higher concentration, adsorption increases slowly and finally reached a plateau level. | ||
| Fig. 1 Adsorption isotherms of (i) pure MB, (ii) MB in the presence of 15 mM Na2HPO4 on kaolinite clay surface. | ||
To know the type of adsorption isotherm, Langmuir and Freundlich isotherm models are most commonly used to correlate the amount adsorbed and the equilibrium concentration for adsorption at solid–liquid interfaces.23 The Langmuir (eqn (1)) and Freundlich (eqn (2)) isotherm equations are expressed as follows:
 | (1) |
 | (2) |
| Adsorption condition | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| qm | b | R2 | a | 1/n | R2 | |
| No electrolyte | 6.93 | 120.33 | 0.99 | 17.37 | 0.41 | 0.81 |
| Na2HPO4 | 15.60 | 45.79 | 0.99 | 19.21 | 0.27 | 0.94 |
3.2 Effect of electrolytes
 | ||
| Fig. 2 Effect of different electrolytes on MB adsorption at 1 mM concentration. Inset shows adsorption in the presence of NaCl. | ||
The electrolyte effect is also analyzed with respect to their ionic strength and shown in Fig. 3. It can be seen that there is a linear relationship between the amount adsorbed and the ionic strength of used electrolytes, with a correlation coefficient of >0.9 in each case. The increasing order of respective slopes for different electrolytes are NaCl < CaCl2 < Na2SO4 < Na2HPO4, which is exactly the same order to that of enhancement of amount adsorbed. These results also indicate that the valance of anion of the electrolyte is an important parameter to enhance the adsorption capacity of dye on the clay surface.
In addition to the electrical behavior, electrolyte solutions may also change the pH of the system, which in turn affect the zeta potential of the clay surface as well as the adsorption behavior. It is evident from the literature that, at high pH (>9) siloxane bond of the clay surface is converted to silanol group and generates more negative zeta potential.28 The Na2HPO4 solution gives an alkaline environment; as a result, siloxane bond converts to silanol. The newly formed silanol group forms hydrogen bond with one amine group of the dye and another amine group forms ionic bond with Si–O−, because of these interactions between clay and dye molecules, the adsorption amount enhances significantly. The mechanism is presented schematically in Scheme 1. While the pH of the solution changes in the presence of electrolyte, there may be a question stability of MB. To support the stability of MB dye at that alkaline pH, some reported literatures mentioned that the methylene blue dye is indefinitely stable in alkaline aqueous solution.29,30 In fact, for the purification of MB, alkali solution is used in the presence of a water insoluble solvent to remove impurities. Additionally, the UV-Vis absorbance of MB solutions in neutral and alkali media (pH 11) was also measured for the comparison and found no significant change in the absorbance maximum (Fig. S1†), little difference in absorbance of two spectra may be because of dilution error.
3.3 FT-IR analysis
The infrared spectroscopy has been widely used to study the structural details of the adsorbed molecules. In this study, after the adsorption process, dye adsorbed clay was analyzed by FT-IR to see the changes. In Fig. 4, pure kaolinite (I) exhibits sharp peaks at 1118, 1067, 1050, 950 cm−1 and very weak bands at ∼3674 and ∼835 cm−1. A peak at 1118 cm−1 in addition to dual peaks at 1067 and 1050 cm−1 absorptions are because of the characteristic vibrational stretching of Si–O–Si group, while peak at 950 cm−1 correspond to Al–OH–Al bending vibrations.32 Moreover, very weak bands at ∼3674 and ∼835 cm−1 are because of –O–H and Si–O stretchings of silanol group of kaolinite.33 | ||
| Fig. 4 FT-IR spectra of pure kaolinite, kaolinite after MB adsorption in the absence and presence of different electrolytes. | ||
Kaolinite upon adsorption with MB in the absence and presence of electrolytes were also further analyzed. In general, MB dye exhibits major spectral absorptions at 1644 and 1587 cm−1 (for ketone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group), 1485 cm−1 (for aromatic C
O group), 1485 cm−1 (for aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch), 1385 cm−1 (for alkyl R–), 1133 cm−1 (for C–N–), 821 cm−1 (for CH
C stretch), 1385 cm−1 (for alkyl R–), 1133 cm−1 (for C–N–), 821 cm−1 (for CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and 666 cm−1 (for C–O–H twist) (Fig. S2†).34 MB in the presence of kaolinite (curve II) shows very low frequency bands between 1317 to 1654 cm−1 region, low intense peaks at 3673, 854, 833, and 813 cm−1, and high intense peaks at 1118, 1050, and 950 cm−1. The presence of bands in the spectral pattern from 1317 to 1654 cm−1 region are because of the presence of MB dye and peaks at 3673, ∼830 cm−1 typically represent the –NH–, CH
C) and 666 cm−1 (for C–O–H twist) (Fig. S2†).34 MB in the presence of kaolinite (curve II) shows very low frequency bands between 1317 to 1654 cm−1 region, low intense peaks at 3673, 854, 833, and 813 cm−1, and high intense peaks at 1118, 1050, and 950 cm−1. The presence of bands in the spectral pattern from 1317 to 1654 cm−1 region are because of the presence of MB dye and peaks at 3673, ∼830 cm−1 typically represent the –NH–, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups of MB respectively, while the rest dominant peaks are for the presence of kaolinite structural groups as stated previously (curve II to VI). Comparing all FT-IR spectra in the presence of different electrolytes (curves III to VI) it has been found that a strong absorption shift (towards lower frequency region) in the presence of electrolyte Na2HPO4 (curve VI). This particular spectrum exhibits intense strong peaks at 3673, 1118, 1022, 941 cm−1 and peaks at ∼850 to 800 cm−1. The high intense pointed peak at 3673 cm−1 confirms the greater exposure of silanol group (Si–O–H) in the kaolinite mixture and is due to intramolecular hydrogen bonding. While curves III to V have shown moderate adsorption shifts when compared to pure kaolinite (curve I) and with lesser shift when compared to curve VI.
C groups of MB respectively, while the rest dominant peaks are for the presence of kaolinite structural groups as stated previously (curve II to VI). Comparing all FT-IR spectra in the presence of different electrolytes (curves III to VI) it has been found that a strong absorption shift (towards lower frequency region) in the presence of electrolyte Na2HPO4 (curve VI). This particular spectrum exhibits intense strong peaks at 3673, 1118, 1022, 941 cm−1 and peaks at ∼850 to 800 cm−1. The high intense pointed peak at 3673 cm−1 confirms the greater exposure of silanol group (Si–O–H) in the kaolinite mixture and is due to intramolecular hydrogen bonding. While curves III to V have shown moderate adsorption shifts when compared to pure kaolinite (curve I) and with lesser shift when compared to curve VI.
The peak shift from 1050 to 1034 cm−1 (difference of 16 cm−1) was observed for curves III to V and peak shift from 1050 to 1022 cm−1 (difference of 28 cm−1) was observed for curve VI along with peak broadening. The observation of this study infers absorption towards lower energy region of the spectra because of intramolecular hydrogen bonding of Si–O stretching vibrations of the tetrahedral sheets exposed upon treatment with different electrolytes.35 In this study, the highest peak shifting was observed for Na2HPO4 treated clay. In general, the hydrogen bonding greatly increases the intensity of the band and moves it to lower frequencies i.e. stronger the hydrogen bonding, broader the absorption band. Intermolecular hydrogen bonding is concentration dependent and gives raise to broad bands. On dilution this behaviour changes and peak intensities will decrease. On the other hand, intramolecular hydrogen bonding is not concentration dependent and no change in intensity occurs upon dilution. In this study, the latter case is obvious (from Fig. 4, curves III to VI) where the intramolecular hydrogen bonding prevailed after the treatment with electrolytes. Since the presence of Na2HPO4 generate alkaline pH of the solution, the siloxane group of clay interacts with the free hydroxyl ions (OH−) and forms silanol group. Formation of hydrogen bond between the nitrogen atom of the MB and silanol group might play a significant role in adsorption process13 and the presence of Si–O–Si and Si–O functional group's presence can be confirmed by vibrational stretching peaks at ∼1060 and ∼830 cm−1 respectively. The FT-IR analysis supports our assumed schematic diagram on MB adsorption onto kaolinite clay surface in Section 3.2.1. Moreover, addition of other electrolytes failed to create the alkaline environment as high as Na2HPO4 and chances of silanol group formation is rare (which is evident from the lower intensity peaks at 3673 cm−1 region in (curve III to V)).
3.4 XRD analysis
The structural changes of clay because of the adsorption of organic molecules can be determined by the XRD analysis. In past few studies, it has been reported that the change in d-spacing is possible while dye molecules adsorbed between layer spacing of the clay.20,36 However, in our study, after the adsorption dye molecules kaolinite clay did not show any significant changes (Fig. S3 and Table S1†). This infers that MB adsorption on the kaolinite clay (in the presence and absence of electrolytes) occurs on the surface of the clay, but not inside the interlayer spacing.3.5 Zeta potential analysis
Zeta potential and pH of the suspension of pure kaolinite clay and that in the presence of different electrolytes (NaCl, Na2SO4, CaCl2, and Na2HPO4 at their respective equilibrium concentrations) are represented in Fig. 5. In the presence of NaCl, Na2SO4, and CaCl2 the suspension pH is almost neutral, whereas in the presence of Na2HPO4 the pH is alkaline. The zeta potentials values are more negative than that of pure kaolinite in the presence of NaCl and Na2SO4, the values are also very close for both electrolytes. In the presence of CaCl2 the negative value of zeta potential decreases compares to that of NaCl and Na2SO4 mainly because of the presence bi-valance cation. Finally, the presence of Na2HPO4 shows highest values of zeta and pH. The higher zeta potential value of kaolinite mainly because of the formation of more silanol groups at higher alkaline pH of the suspension, as mentioned in the Section 3.3, which in turn also helps to enhance the adsorption capacity.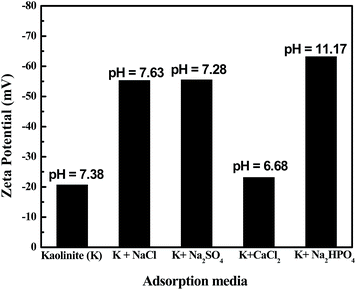 | ||
| Fig. 5 Zeta potential and pH of the kaolinite clay at different adsorption conditions [NaCl (100 mM), CaCl2 (30 mM), Na2SO4 (25 mM), Na2HPO4 (15 mM)]. | ||
4. Conclusions
The main findings of this study can be summarized as follows:In the presence of electrolyte solutions, the MB adsorption capacity of the kaolinite clay surface is significantly enhanced. The adsorption enhancement ability of four electrolytes of different valance studied here (NaCl, Na2SO4, CaCl2, and Na2HPO4) on a constant MB concentration follow the following order: NaCl < CaCl2 < Na2SO4 < Na2HPO4. The maximum concentration of electrolyte required for saturation level of adsorption follow the reverse order NaCl (100 mM) > CaCl2 (30 mM) > Na2SO4 (25 mM) > Na2HPO4 (15 mM). In the presence of Na2HPO4, the adsorption capacity of kaolinite is ∼127% higher than that in the absence of electrolyte. In the presence of Na2HPO4, the negative zeta potential on kaolinite surface increases and the repulsive force among the cationic dye molecules after the adsorption decreases. Hence, both zeta potential and repulsive force are responsible parameters for the enhancement of dye adsorption. The increase in negative zeta potential of kaolinite in the presence of Na2HPO4 is mainly because of alkaline medium, which helps the formation of silanol group on the clay surface. Finally, the synergistic effect of pH and electrolyte is very important for the adsorption enhancement of MB. Since clay is abundantly available, cheap adsorbent, and the adsorption capacity of clay can be enhanced significantly by using inexpensive electrolytes, this technique may be very useful for the remediation of dye contaminated surface water.
Acknowledgements
One of the authors K. M thanks Director, National Institute of Technology, Rourkela, India, for a Post Doctoral Fellowship to pursue this work.References
- M. Stylidi, D. I. Kondarides and X. E. Verykios, Appl. Catal., B, 2004, 47, 189 CrossRef CAS PubMed.
- G. Walker, L. Hansen, J.-A. Hanna and S. Allen, Water Res., 2003, 37, 2081 CrossRef CAS.
- M. T. Uddin, M. Rukanuzzaman, M. M. R. Khan and M. A. Islam, J. Environ. Manage., 2009, 90, 3443 CrossRef PubMed.
- S. Gupta and B. Babu, Bioresour. Technol., 2009, 100, 5633 CrossRef CAS PubMed.
- R. Gong, X. Zhang, H. Liu, Y. Sun and B. Liu, Bioresour. Technol., 2007, 98, 1319 CrossRef CAS PubMed.
- D. Suteu and D. Bilba, Acta Chim. Slov., 2005, 52, 73 CAS.
- M. J. Iqbal and M. N. Ashiq, J. Hazard. Mater., 2007, 139, 57 CrossRef CAS PubMed.
- D. Suteu, A. Nacu and G. Cristian, Cellul. Chem. Technol., 2001, 35, 451 CAS.
- A. L. Abdullah, M. M. Salleh, M. S. Mazlina, M. M. M. Noor, M. Osman, R. Wagiran and S. Sobri, Int. J. Eng. Technol., 2005, 2, 8 Search PubMed.
- U. Filipkowska, E. Klimiuk, S. Grabowski and E. Siedlecka, Pol. J. Environ. Stud., 2002, 11, 315 CAS.
- S. D. Abayazeed and E. El-Hinnawi, Am. J. Appl. Sci., 2011, 8, 1282 CrossRef CAS.
- K. G. Bhattacharyya and S. S. Gupta, Adv. Colloid Interface Sci., 2008, 140, 114 CrossRef CAS PubMed.
- M. Khraisheh, M. Al-Ghouti, S. Allen and M. Ahmad, Water Res., 2005, 39, 922 CrossRef CAS PubMed.
- A. Gürses, Ç. Doğar, M. Yalçın, M. Açıkyıldız, R. Bayrak and S. Karaca, J. Hazard. Mater., 2006, 131, 217 CrossRef PubMed.
- R. S. Heughebaert, Clay Miner., 1992, 27, 91 Search PubMed.
- A. Aznar, B. Casal, E. Ruiz-Hitzky, I. Lopez-Arbeloa, F. Lopez-Arbeloa, J. Santaren and A. Alvarez, Clay Miner., 1992, 27, 101 CAS.
- ASTM, Standard test method for methylene blue index of clay, ASTM C837-99, 2003 Search PubMed.
- APIP, Recommended Practice Standard Procedure for Testing Drilling Fluids, American Petroleum Institute, Dallas, Tx., 1st edn, 1982, p. 48 Search PubMed.
- D. Ghosh and K. G. Bhattacharyya, Appl. Clay Sci., 2002, 20, 295 CrossRef CAS.
- P. T. Hang and G. Brindley, Clays Clay Miner., 1970, 18, 203 Search PubMed.
- H. Tahir, M. Sultan and Z. Qadir, Int. J. Chem., 2013, 5, 19 CAS.
- R. Atkin, V. Craig, E. Wanless and S. Biggs, J. Colloid Interface Sci., 2003, 266, 236 CrossRef CAS.
- N. R. Biswal and S. Paria, Ind. Eng. Chem. Res., 2010, 49, 7060 CrossRef CAS.
- S. Paria and P. K. Yuet, Ind. Eng. Chem. Res., 2006, 45, 712 CrossRef CAS.
- R. G. Chaudhuri and S. Paria, J. Colloid Interface Sci., 2009, 337, 555 CrossRef CAS PubMed.
- R. G. Chaudhuri, S. Sunayana and S. Paria, Soft Matter, 2012, 8, 5429 RSC.
- R. G. Chaudhuri and S. Paria, J. Colloid Interface Sci., 2014, 413, 24 CrossRef PubMed.
- K. K. Unger, Porous silica: its properties and use as support in column liquid chromatography, Elsevier, 1979, p. 86 Search PubMed.
- A. Mills, D. Hazafy, J. Parkinson, T. Tuttl and M. G. Hutchings, Dyes Pigm., 2011, 88, 149 CrossRef CAS PubMed.
- D. C. Abbott, Analyst, 1962, 87, 286 RSC.
- C.-K. Lee, S.-S. Liu, L.-C. Juang, C.-C. Wang, K.-S. Lin and M.-D. Lyu, J. Hazard. Mater., 2007, 147, 997 CrossRef CAS PubMed.
- N. Worasith, B. Goodman, J. Neampan, N. Jeyachoke and P. Thiravetyan, Clay Miner., 2011, 46, 539 CrossRef CAS PubMed.
- R. A. Da Silva and D. J. Guerra, J. Chil. Chem. Soc., 2013, 58, 1517 CrossRef CAS.
- L. W. Low, T. T. Teng, A. Ahmad, N. Morad and Y. S. Wong, Water, Air, Soil Pollut., 2011, 218, 293 CrossRef CAS.
- J. Theil, D. Tsu, M. Watkins, S. Kim and G. Lucovsky, J. Vac. Sci. Technol., A, 1990, 8, 1374 CAS.
- G. Lv, Z. Li, W.-T. Jiang, P.-H. Chang, J.-S. Jean and K.-H. Lin, Chem. Eng. J., 2011, 174, 603 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03534a |
| This journal is © The Royal Society of Chemistry 2015 |


