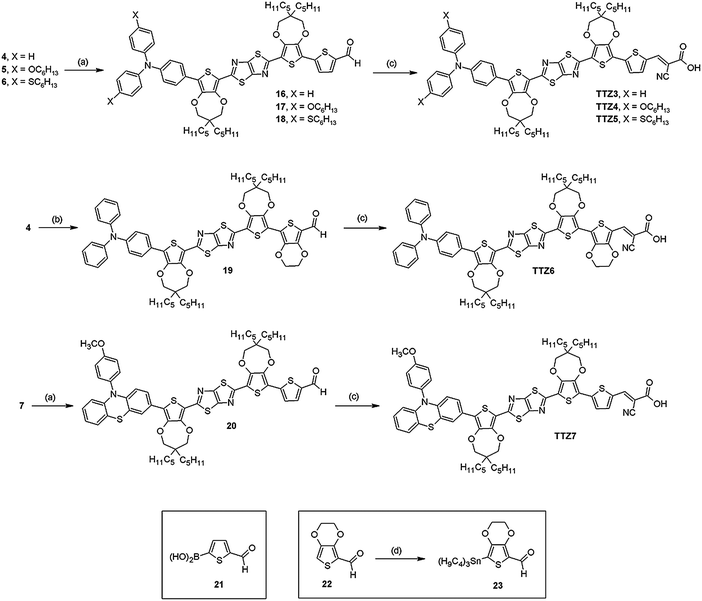
Thiazolo[5,4-d]thiazole-based organic sensitizers with strong visible light absorption for transparent, efficient and stable dye-sensitized solar cells†
Alessio Dessìab,
Massimo Calamanteab,
Alessandro Mordiniab,
Maurizio Peruzzinia,
Adalgisa Sinicropic,
Riccardo Basosic,
Fabrizia Fabrizi de Bianic,
Maurizio Taddeic,
Daniele Colonnad,
Aldo di Carlode,
Gianna Reginato*a and
Lorenzo Zani*a
aIstituto di Chimica dei Composti Organometallici (CNR-ICCOM), Via Madonna del Piano 10, 50019 Sesto Fiorentino (FI), Italy. E-mail: gianna.reginato@iccom.cnr.it; lorenzo.zani@iccom.cnr.it; Fax: +39 055 5225203; Tel: +39 055 5225245
bDipartimento di Chimica “U. Schiff”, Università degli Studi di Firenze, Via della Lastruccia 13, 50019 Sesto Fiorentino (FI), Italy
cDipartimento di Biotecnologie, Università degli Studi di Siena, Chimica e Farmacia, Via A. Moro 2, 53100 Siena, Italy
dDyepower Consortium, Viale Castro Pretorio 122, 00185 Rome, Italy
eCenter for Hybrid and Organic Solar Energy (C.H.O.S.E.), Università degli Studi di Roma “Tor Vergata”, Via del Politecnico 1, 00133 Rome, Italy
First published on 31st March 2015
Abstract
A small set of thiazolo[5,4-d]thiazole-based D–π–A organic dyes, endowed with bis-pentylpropylenedioxythiophene (ProDOT) moieties in the π-spacer, was designed with the aid of computational analysis, synthesized and characterized. The presence of bulky and electronrich ProDOT groups beside the electron poor thiazolothiazole unit induced optimal physico-chemical properties, including broad and intense visible light absorption. As a consequence, the dyes were particularly suitable for application in thin layer dye-sensitized solar cells (TiO2 thickness: 3.0–6.5 μm). Small-scale (0.25 cm2) devices prepared using standard materials and fabrication techniques gave power conversion efficiencies up to 7.71%, surpassing those obtained with two different reference dyes. Transparent larger area cells (3.6 cm2) also showed good η values up to 6.35%, not requiring the use of a co-adsorbent, and retained their initial efficiency over a period of 1000 h storage at 85 °C. These results make this new family of organic sensitizers promising candidates for successful application in the production of efficient and stable transparent DSSCs for building-integrated photovoltaics.
Introduction
Since their discovery in 1991,1 dye-sensitized solar cells (DSSCs) have been widely regarded as a potential alternative to traditional silicon-based photovoltaic devices.2,3 In a DSSC, light harvesting takes place at the photoanode, constituted by a thin layer of a wide-band gap inorganic semiconductor (most often TiO2) sensitized with a suitable dye: therefore, the choice of sensitizer plays a crucial role in determining the overall performances of the device.Traditionally, DSSCs with high power conversion efficiencies (η) were first obtained with ruthenium metal-organic sensitizers,4 whose performances were later surpassed by porphyrin dyes5 and, most recently, lead-containing perovskites.6 Fully organic sensitizers have also been employed, and efficiencies higher than 10%,7–9 sometimes even coupled with excellent stabilities,10 have been observed in such cases. The latter class of dyes presents some potential advantages compared to the other sensitizers, such as the possibility of not using precious or toxic metals, the ability to modify molecular properties by flexible synthetic procedures and the efficient harvesting of sunlight due to high molar absorptivities.11
In particular, organic dyes with superior light absorption ability would be especially suitable for the sensitization of thin TiO2 layers,12 possibly useful for the development of solid-state DSSCs,13 but also for the construction of colored, transparent photovoltaic modules. The latter could find optimal application in the context of building-integrated photovoltaics (BIPV),14 currently considered one of the most appealing opportunities for DSSC commercialization.15
Another key point for a wide application of DSSCs has to deal with the simplification of fabrication techniques. It is well known that the application of surface treatments on both the conductive glass and the inorganic semiconductor, the consecutive deposition of multiple TiO2 layers of different average particle size and shape,16 or the introduction of several additives and co-adsorbents both in the sensitizing baths and cell electrolytes10 are often required to obtain high efficiency. The discovery of new sensitizers able to give good performances under simplified conditions would allow to reduce the economic and environmental costs of DSSCs,17 thus helping to make this technology more sustainable.
In recent years, both we18 and others19 introduced new DSSC dyes featuring fused thiazole heterocycles as central electron withdrawing units. Among our compounds, sensitizer TTZ1 (Fig. 1), having a thiazolo[5,4-d]thiazole (TzTz) core,20 provided the best performances, with a maximum η of 3.53% obtained in the presence of chenodeoxycholic acid (CDCA) as a co-adsorbent.18a We reasoned that the low efficiency observed for TTZ1 could be attributed to a combination of factors, such as its limited light harvesting ability, its scarce solubility in most organic solvents and its tendency to form aggregates on TiO2 surface, requiring the use of CDCA to improve photocurrents. Based on these considerations, we designed a new series of TzTz-containing sensitizers (see Fig. 1, TTZ3–7), with the precise aim to increase their visible light absorption and improve their solubility compared to TTZ1.
We supposed that replacement of the simple hexyl chains present in TTZ1 with two alkoxy substituents could reduce the aromatic stabilization of the thiophene rings, thus slightly increasing the HOMO energies and decreasing the HOMO–LUMO gaps of the resulting molecules, ultimately leading to a red-shift of their absorption spectra. Moreover, a spectral red-shift and an improvement in light harvesting were expected by alternating the electronrich thiophene moieties with the electron withdrawing thiazolothiazole and cyanoacrylic units.21 Finally, placement of further alkyl chains on the sensitizers backbone was regarded as a way to improve their solubility, which was considered beneficial both for their synthesis as well as their purification.
For these reasons, we replaced the 3-hexylthiophene units of TTZ1 with bulkier and more electronrich bis-pentylpropylenedioxythiophene (ProDOT) moieties (TTZ3, Fig. 1).22 The design of such scaffold was supported by an accurate DFT computational analysis. We also modified the terminal triphenylamine group by introducing different substituents in para-position (TTZ4–5). The preparation and application of compounds TTZ3–5 have been already reported by us in a recent preliminary communication.23 To further modulate the electronic properties of the dyes and influence their photovoltaic performances, we decided to alter the conjugate portion of the compounds by inserting an electronrich ethylenedioxythiophene (EDOT) ring in place of the terminal thiophene present in TTZ3–5 (TTZ6), and also to modify the terminal donor unit by replacing the triarylamine group with a phenothiazine moiety (TTZ7).
In addition to the previously reported data, herein we will disclose a full account describing the computational validation of compounds TTZ3–7, their detailed synthesis as well as their complete characterization. We will also report their use as sensitizers for thin-layer DSSCs under a variety of conditions (opaque/transparent semiconductor layers, presence/absence of additives, different layer thicknesses and surface areas, different electrolytes), and compare their performances with those of different reference sensitizers. Finally, the results of stability studies conducted under controlled conditions, as well as those of electrochemical impedance spectroscopy investigations, will be discussed.
Results and discussion
Computational analysis
Before starting our synthetic efforts, we analysed compounds TTZ3–7 computationally. In the calculations, the alkyl chains present on the molecules were replaced by simple methyl groups to reduce the computational burden, without affecting the length and nature of the conjugate system.Initially, structures were optimized in vacuo by means of DFT calculations at the B3LYP/6-31G* level;24 the energy and shape of their frontier molecular orbitals (FMOs) were then compared with those previously found for dye TTZ1, which was used as a reference. Computed values of the HOMO–LUMO energy gaps for the TTZ3–7 structures having both ProDOT rings in the most stable “chair” conformation are reported in Fig. 2, while the wave function plots of the FMOs are reported in Fig. S1 (ESI†).
Dye TTZ3 showed a reduced HOMO–LUMO gap compared to TTZ1 (2.11 eV vs. 2.17 eV), mostly due to HOMO destabilization. The HOMO energy increased even further and the frontier orbital gap decreased (1.95 eV) when the terminal triphenylamine unit was decorated with alkoxy substituents (TTZ4) to increase its electron donating character. In the case of dye TTZ5, alkylthio substituents were introduced on the donor moiety, since it was shown that their use could lead to DSSCs with improved Voc and η compared to the oxygenated counterparts (for a discussion, see below).25 The presence of such functional groups had a similar, but smaller effect on the HOMO energy, resulting in a FMO gap of 2.00 eV. Compound TTZ7, bearing a phenothiazine donor moiety,26 had a HOMO–LUMO gap comparable to that found for dye TTZ3, with a value of 2.09 eV. Finally, in the case of compound TTZ6, the presence of an EDOT group on the terminal portion made the molecule more electronrich, in turn causing a significant LUMO destabilization without a big change of the HOMO energy. As a result, the FMO gap in this compound was larger than in dyes TTZ3–5,7, and closer to that found for TTZ1 (2.17 vs. 2.18 eV). For all compounds, orbital shapes were similar, with the HOMO − 1 distributed along the entire conjugated system and the HOMO and LUMO mostly localized on the donor and acceptor moieties, respectively (Fig. S1†).
To learn more on the electronic transitions of the new organic sensitizers, the absorption maxima (λamax), oscillator strengths (f) and vertical excitation energies (Eexc) in the most stable conformation were assessed by TD-DFT calculations in THF at the CAM-B3LYP/6-31G*27 level (Table S1†). For all dyes the excitation process in the visible region results mostly from mixed HOMO → LUMO and HOMO − 1 → LUMO transitions. Interestingly, although TTZ6 was the compound with the highest computed HOMO–LUMO gap, its excitation comprised a larger H → L fraction compared to the other dyes, resulting in a slightly red-shifted absorption maximum. In agreement with our expectations, computational results indicated that the new dyes should all have absorption maxima higher than 500 nm, red-shifted of approx. 20–30 nm relative to TTZ1; in addition, the higher oscillator strengths found for TTZ3–7 suggested the possibility of more intense light absorption.
Synthesis of compounds TTZ3–7
The starting point of the synthesis of dyes TTZ3–7 was constituted by aldehyde 1, which was prepared in four steps following a reported procedure.28 Compound 1 was converted to the corresponding thiazolo[5,4-d]thiazole (2) by reaction with dithiooxamide under microwave activation (Scheme 1): initially, we carried out this transformation under the optimized conditions already established in our previously reported MW-assisted synthesis of thiazolothiazoles (nitrobenzene solution, 150 °C, 30 min).29 However, we quickly realized that, due to the presence of the long alkyl chains on the ProDOT moieties, the product would not precipitate from the reaction mixture, thus complicating its separation from nitrobenzene. For this reason, we changed the solvent to n-BuOH30 (facilitating chromatographic purification of 2) and briefly optimized reaction temperature and time, ultimately obtaining the desired heterocyclic product in moderate yield.After electrophilic halogenation with N-iodosuccinimide, the resulting bis-iodide 3 was subjected to Pd-catalyzed Suzuki cross-coupling with boronic acids/esters 8–9, 12 and 15 to install the donor moieties. To minimize formation of the symmetrical double-coupling products, a stoichiometric amount of boronic reagent had to be employed, and the reaction had to be stopped before reaching full conversion, resulting in low yields of products 4–7; despite that, a significant amount of unreacted diiodide 3 could usually be recovered after chromatographic purification for further reuse. Due to the lower reactivity of boronic esters 10 and 15, an alternative protocol using [Pd(dppf)Cl2] as the catalyst and KF as the base was employed instead of the classical procedure with Pd(PPh3)4.
Whereas boronic acid 8 is commercially available, and ester 9 was obtained via a reported procedure,31 compound 12 was prepared by the route shown in Scheme 1. First, Pd-catalyzed Buchwald–Hartwig reaction between 1-bromo-4-hexylthiobenzene32a and aniline afforded triarylamine 10, which was then brominated with N-bromosuccinimide (NBS) to yield compound 11.32b Finally, conversion of the latter to the corresponding boronic ester 12 was accomplished via Pd-catalyzed borylation, which proceeded with higher yield than the previously reported Li-mediated procedure.32b
Reagent 15 was also prepared through a bromination/catalytic borylation sequence starting from known phenothiazine 13.33
However, initial electrophilic halogenation with typical reagents such as NBS and Br2 under various conditions took place with limited success due to either lack of reactivity, or formation of bis-brominated derivatives. For this reason, we examined alternative reaction conditions, and ultimately employed a sub-stoichiometric amount of pyridinium bromide perbromide34 to obtain a mixture of 13 and 14 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio, which could be used as such in the following step. Pd-catalyzed borylation of bromide 14 with bis(pinacolato)diboron then took place uneventfully, producing boronic ester 15 in good yield after chromatographic separation from unreacted 13.
3 molar ratio, which could be used as such in the following step. Pd-catalyzed borylation of bromide 14 with bis(pinacolato)diboron then took place uneventfully, producing boronic ester 15 in good yield after chromatographic separation from unreacted 13.
After preparation of intermediates 4–7, the synthesis of dyes TTZ3–7 was completed by introduction of the acceptor moiety through a cross-coupling/Knoevenagel condensation sequence, with both processes proceeding in good to high yields (Scheme 2). In the case of compounds TTZ3–5 and TTZ7 the first reaction was a Suzuki coupling of iodides 4–7 with commercial boronic acid 21: such transformation was activated by microwaves to shorten reaction times and limit the formation of by-products (for example deriving from protodehalogenation reactions). Stille cross-coupling of intermediate 4 with EDOT-containing stannane 23 was employed instead for dye TTZ6. Stannane 23 was prepared starting from known aldehyde 22 (ref. 35) by protection of the carbonyl group with trimethylorthoformate followed by lithiation of the resulting dimethylacetal and treatment with tributyltin chloride; at the end of the reaction, work-up with an aqueous solution of KHSO4 led to deprotection of the formyl group, allowing isolation of compound 23.
Characterization of dyes TTZ3–7
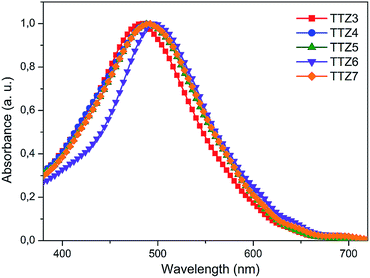
In THF solution, all dyes showed broad and intense absorptions in the visible region (Fig. 3), with maxima in the 510–521 nm range, thus displaying a bathochromic shift of ca. 40–50 nm compared to TTZ1.18a In agreement with the results of our computational analysis (see above), dyes TTZ4 and TTZ6 showed the most red-shifted absorption maxima; more generally, experimental excitation energies were quite close to the calculated values, with differences of at most 0.1 eV. | ||
| Fig. 3 UV-Vis spectra of dyes TTZ3–7 in THF solution. Concentrations: TTZ3, 8.47 × 10−6 M; TTZ4, 8.89 × 10−6 M; TTZ5, 8.96 × 10−6 M; TTZ6, 7.61 × 10−6 M; TTZ7, 9.57 × 10−6 M. | ||
All dyes exhibited fluorescence in THF solution: therefore, optical band gaps (E0–0) could be obtained from the intersection of the normalized absorption and emission spectra (Fig. S2†), and were comprised in the 2.14–2.25 eV range (Table 1), with TTZ4 and TTZ6 having the smallest E0–0 values. Such values were in good agreement with those derived from the corresponding Tauc plots,36 which were found to be in the 2.12–2.16 eV range and in the same relative order (Fig. S3†).
| Dye | λmax absa [nm] (ε [M−1 cm−1]) | λmax ema [nm] | λmax abs. on TiO2 [nm] | Eoxb [V] | E*oxc [V] | E0–0d [eV] | Γ [107 mol cm−2] |
|---|---|---|---|---|---|---|---|
| a THF solution.b Potential vs. NHE.c Calculated from Eox − E0–0.d Estimated from the intersection of normalized absorption and emission spectra. | |||||||
| TTZ3 | 510 (81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400) 400) |
587 | 484 | 1.02 | −1.23 | 2.25 | 0.99 |
| TTZ4 | 518 (86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) 600) |
601 | 491 | 0.77 | −1.42 | 2.19 | 1.08 |
| TTZ5 | 510 (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
573 | 487 | 0.91 | −1.33 | 2.24 | 1.19 |
| TTZ6 | 521 (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), 548 (85 500), 548 (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
636 | 498 | 0.93 | −1.21 | 2.14 | 1.25 |
| TTZ7 | 513 (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) 700) |
596 | 492 | 0.85 | −1.37 | 2.22 | 1.75 |
When the dyes were attached to nanocrystalline TiO2, they exhibited slightly broader and blue-shifted absorption spectra (21–27 nm), probably due to deprotonation of the carboxylic acid group on the semiconductor surface (Fig. 4).37 The amount of dyes attached to TiO2 was measured through the desorption method, by exposing electrodes sensitized with TTZ3–7 (similar to those later used for DSSC fabrication) to a 0.1 M KOH solution in THF/MeOH until complete discoloration, and measuring the absorbance of the resulting solution. The highest density was measured for TTZ7, but all dyes exhibited similar values in the 0.99–1.75 × 10−7 mol cm−2 range.
Ground-state oxidation potentials (Eox) of all compounds were measured by means of cyclic voltammetry in CH2Cl2 solution (Fig. S4†). The strong electrondonating character resulting from introduction of the alkoxy groups on the triarylamine portion of TTZ4 was reflected by its relatively low Eox value (0.77 eV vs. NHE); interestingly, also the presence of the terminal phenothiazine unit had a similar effect, with TTZ7 displaying a much lower oxidation potential compared to TTZ3. However, all the dyes had Eox values positive enough to ensure regeneration by the iodide/triiodide redox shuttle.38 Excited state oxidation potentials were obtained from the expression E*ox = Eox − E0–0, and were always found to be more negative than the conduction band edge of TiO2 (approx. −0.5 V vs. NHE), confirming the possibility of smooth electron injection from the dye to the semiconductor during solar cell operation.
Small-scale photovoltaic measurements
After characterization, compounds TTZ3–7 were employed as sensitizers in the construction of small-scale test DSSCs (0.25 cm2 active surface, see Fig. 6a), aiming to evaluate their general level of performance and determine which one had the most promising photovoltaic properties.In view of the very high molar extinction coefficients, suggesting the possibility of efficient light-harvesting, we reasoned that the new dyes could be conveniently used in DSSCs having a thinner TiO2 layer compared to typical devices. For this reason, two series of cells were assembled: a first series with a transparent photoanode of 5.5 μm thickness (obtained with commercial Dyesol 18NR-T paste), and a second series with an opaque electrode of 6.5 μm thickness (Dyesol 18NR-AO).
We decided to keep device fabrication as simple as possible, as we reasoned that the efficiency data obtained under such simplified conditions could serve as a useful starting point to assess the potential of the new dyes in view of possible, more challenging, large-scale applications. In particular, no blocking layer of TiO2 was deposed either on the conductive glass substrate or on the nanocrystalline semiconductor layer by treatment with aq. TiCl4, and no light-scattering layer was employed in photoanode fabrication. Furthermore, differently from previous studies on thin-layer DSSCs,39,40 a commercial electrolyte solution based on the I−/I3− redox couple (Dyesol HPE) was used in the cells, since it usually gives optimal results with ruthenium sensitizers and is therefore still the most used for practical applications such as BIPV.
For both types of devices, performances obtained with the new sensitizers were compared with those of standard organic dye D5 (ref. 41 and 42) and Ru-based sensitizer Z907 (Fig. S5†).43,44 Typical J/V curves measured on the transparent solar cells are shown in Fig. 5a, while the corresponding IPCE spectra are displayed in Fig. 5b and the relevant photovoltaic parameters are reported in Table 2.
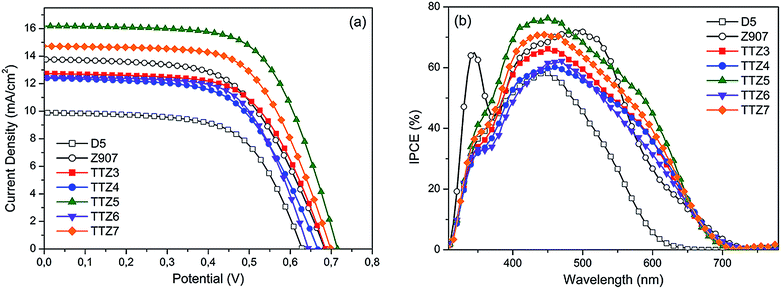 | ||
| Fig. 5 Typical J/V curves (a) and IPCE spectra (b) for transparent cells built with dyes D5, Z907 and TTZ3–7. | ||
| Dye | Transparent DSSCsb | Opaque DSSCsc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CDCAd | Jsc [mA cm−2] | Voc [V] | FF [%] | η [%] | CDCAd | Jsc [mA cm−2] | Voc [V] | FF [%] | η [%] | |
| a Average values of three devices; active surface area 0.25 cm2.b TiO2 layer thickness 5.5 μm.c TiO2 layer thickness 6.5 μm.d “−“: without CDCA; “+”: with 1 mM CDCA added in the sensitizing bath (0.1 mM dye in THF). | ||||||||||
| D5 | − | 9.60 | 0.624 | 63 | 3.78 | − | 11.04 | 0.619 | 58 | 3.99 |
| Z907 | − | 13.50 | 0.686 | 60 | 5.51 | − | 13.85 | 0.687 | 58 | 5.61 |
| TTZ3 | − | 12.79 | 0.687 | 61 | 5.41 | − | 13.65 | 0.671 | 60 | 5.45 |
| + | 15.62 | 0.697 | 60 | 6.55 | + | 16.52 | 0.683 | 58 | 6.54 | |
| TTZ4 | − | 12.18 | 0.669 | 60 | 4.85 | − | 12.99 | 0.665 | 57 | 4.93 |
| + | 14.27 | 0.692 | 59 | 5.85 | + | 15.18 | 0.675 | 55 | 5.71 | |
| TTZ5 | − | 16.20 | 0.716 | 63 | 7.39 | − | 16.05 | 0.721 | 60 | 6.91 |
| + | 16.59 | 0.717 | 59 | 7.08 | + | 18.33 | 0.709 | 59 | 7.71 | |
| TTZ6 | − | 12.29 | 0.646 | 63 | 5.01 | − | 13.00 | 0.644 | 56 | 4.70 |
| + | 10.68 | 0.640 | 57 | 3.90 | + | 13.86 | 0.646 | 56 | 5.03 | |
| TTZ7 | − | 14.99 | 0.686 | 62 | 6.35 | − | 15.26 | 0.680 | 59 | 6.08 |
| + | 15.97 | 0.694 | 60 | 6.61 | + | 17.22 | 0.689 | 58 | 6.94 | |
In the case of transparent cells, dyes TTZ3–7 gave efficiency values between 4.85 and 7.39%, with TTZ5 and TTZ7 being the best two sensitizers. Remarkably, all new thiazolo[5,4-d]thiazole dyes gave better efficiencies than standard organic dye D5, and the two most efficient compounds surpassed even the performance provided by ruthenium sensitizer Z907 (η 6.35–7.39% vs. 5.51%). This result was particularly significant, since Z907 is one of the sensitizers of choice for applications in BIPV.45 All new compounds gave broad IPCE spectra with onsets around 700 nm (Fig. 5b), in agreement with their UV-Vis spectra recorded on TiO2: in particular, the IPCE of dye TTZ5 was superior to Z907 in the 370–480 and 550–660 nm regions, consistent with its higher photocurrent.
The improved performance of dye TTZ5 compared to the other sensitizers could be attributed to the presence of an alkylthio-substituted triphenylamine moiety as the donor portion of the molecule. A conjugated dye bearing such donor group was investigated in detail by Berlinguette et al. and, as mentioned above, was found to induce superior photocurrent and photovoltage in DSSCs compared to its oxygenated analogue:25 such effect was attributed to a 25-fold enhancement of the dye regeneration rate constant which, according to the authors, could either be due to formation of an adduct between iodide and the “soft” sulphur atoms, or simply result from the greater HOMO coefficients found on the S atoms compared to the O atoms. The latter observation is supported also by our findings (compare the HOMO isodensity plots of TTZ4 and TTZ5 in Fig. S1,† and consider the higher Jsc and Voc recorded for dye TTZ5).
The lower efficiencies recorded with the other dyes could be due to a combination of several factors, including lower light-harvesting efficiency (TTZ3, lowest molar absorptivity and dye density on TiO2), insufficient dye regeneration (TTZ4, highest Eox among all dyes) and increased recombination (TTZ6, larger HOMO coefficients on the terminal part of the molecule, see Fig. S1†).
Data obtained for opaque cells were generally consistent with those recorded for transparent devices (Table 2 and Fig. S6 and 7†), with TTZ5,7 being once again the two best sensitizers, albeit with slightly reduced efficiency values (η 6.08–6.91%). Short-circuit current, open-circuit voltage and fill factor values were similar in the two classes of cells, reflecting the use of analogous fabrication conditions. The relatively low fill factors observed for all cells (55–63%) were likely due to the small thickness of the TiO2 layer employed, combined with the absence of a blocking layer, probably favouring electron recombination through direct contact between the conductive substrate and the electrolyte solution.
To verify if the tridimensional arrangement of alkyl chains on the ProDOT moieties22 was sufficient to suppress aggregation of the new dyes on TiO2 surface, we also built two series of cells for which photoanode staining was carried out in the presence of 1 mM chenodeoxycholic acid (CDCA, Table 2 and Fig. S8 and 9†). In both device classes, an increase in Jsc was observed for all sensitizers, with the sole exception of TTZ6, perhaps due to the steric bulk of its terminal EDOT ring (minimizing aggregation even in the absence of CDCA), while the largest enhancement was displayed by dye TTZ3 (+21–22%). This time, the best result was provided by TTZ5-containing opaque cells, which gave an average efficiency of 7.71% with a remarkable photocurrent of 18.33 mA cm−2.
Fabrication and characterization of strip DSSCs
Following the promising results obtained with small-scale DSSCs (0.25 cm2), we decided to build larger area (3.6 cm2) strip cells (Fig. 6b), aiming to investigate how the increase in active surface could affect device efficiency and stability, in view of a possible scale-up of the system. Accordingly, we also introduced some changes in device fabrication. First, electrodes were prepared with two different thicknesses of transparent TiO2, namely 3 and 5 μm, in order to minimize semiconductor employment and enhance transparency; secondly, a different commercial electrolytic solution (Dyesol HSE) was used, still based on the I−/I3− redox couple, which is designed to improve device stability at the cost of maximum performance. Finally, only dyes TTZ3–5 were used in these experiments, due to their easier preparation procedure. | ||
| Fig. 6 (a) Small-scale dye-sensitized solar cells (active area 0.25 cm2) and (b) strip DSSCs (active area 3.6 cm2) used in this study. | ||
Again, for each electrode thickness, two series of cells were prepared, either by addition of CDCA in the sensitizing bath or not. The well-known organic dye D35 (Fig. S5†) was selected as a reference,46 since this compound has recently been established worldwide as a reference organic sensitizer for DSSCs, due to its good performances coupled with high transparency and excellent stability.47 Typical J/V curves obtained for the CDCA-free and CDCA-containing strip cells are shown in Fig. 7a and b, respectively, while the relevant photovoltaic parameters are reported in Tables 3 and 4.
 | ||
| Fig. 7 Typical J/V curves for transparent strip cells built with dyes D35 and TTZ3–5 in the absence (a) and in the presence (b) of CDCA. Hollow symbols: 3 μm devices; full symbols: 5 μm devices. | ||
| Dye | Thickness [μm] | Jsc [mA cm−2] | Voc [V] | FF [%] | η [%] |
|---|---|---|---|---|---|
| a Average values of two devices; active surface area 3.6 cm2. | |||||
| D35 | 3 | 9.93 | 0.758 | 54 | 4.06 |
| TTZ3 | 3 | 14.65 | 0.693 | 53 | 5.40 |
| 5 | 15.70 | 0.678 | 52 | 5.58 | |
| TTZ4 | 3 | 13.14 | 0.682 | 52 | 4.70 |
| 5 | 13.51 | 0.667 | 52 | 4.71 | |
| TTZ5 | 3 | 15.78 | 0.732 | 53 | 6.20 |
| 5 | 17.00 | 0.705 | 53 | 6.35 | |
| Dye | Thickness [μm] | Jsc [mA cm−2] | Voc [V] | FF [%] | η [%] |
|---|---|---|---|---|---|
| a Average values of two devices; active surface area 3.6 cm2; 1.0 mM CDCA was added to the electrode sensitization bath. | |||||
| D35 | 3 | 9.52 | 0.754 | 55 | 3.93 |
| TTZ3 | 3 | 13.80 | 0.696 | 47 | 4.52 |
| 5 | 16.31 | 0.667 | 50 | 5.48 | |
| TTZ4 | 3 | 12.62 | 0.690 | 52 | 4.55 |
| 5 | 14.31 | 0.677 | 52 | 5.04 | |
| TTZ5 | 3 | 15.29 | 0.722 | 54 | 6.03 |
| 5 | 16.38 | 0.711 | 50 | 5.88 | |
Although DSSCs with a thicker TiO2 layer gave slightly improved performances compared to those with a thinner electrode, differences in efficiency were rather small. Predictably, thicker devices produced a higher photocurrent as a result of the larger amount of dye adsorbed on the anode, but at the same time their open-circuit voltages were smaller, probably due to higher electron recombination. Fill factors of these devices were generally lower than those of small-scale cells due to the higher series resistance usually observed for larger DSSCs.48 However, it was really remarkable that all the three dyes TTZ3–5 yielded more efficient devices than standard sensitizer D35, with the best result provided once again by TTZ5 (6.35% average efficiency for the 5 μm device). The superior efficiencies were mostly due to the higher photocurrents of the thiazolo[5,4-d]thiazole-containing dyes compared to D35; such difference could be explained considering their wider (and in the case of TTZ5 also higher) IPCE spectra, see Fig. S10–12.†
To our surprise, and in contrast to what was observed in the case of small-scale cells, the effect of CDCA on the performance of the larger-area devices (Table 4 and Fig. 7b) was that of giving lower efficiencies compared to the CDCA-free strip cells, with the sole exception of the 5 μm device built with dye TTZ4. In particular, no general improvement in photocurrent was observed in this second series of cells, highlighting a lower tendency of the dyes to aggregate under these conditions. Accordingly, we tentatively assume that when the standard 0.1 mM dye solution is used to sensitize the larger electrodes, a more homogeneous distribution of the sensitizer is achieved, minimizing stacking and other aggregation phenomena and thus eliminating the potential benefits brought by the co-adsorbent.
At this stage, the stability of the strip cells fabricated with dyes TTZ3–5 (Fig. 8) was also tested. A new series of 3 μm-thick devices was built and their power conversion efficiencies measured under standard illumination over a period of approx. 1000 h, during which they were stored in the dark at 85 °C. All DSSCs showed excellent stability during this period of time, with final efficiencies comparable to the initial ones (for TTZ4-5 a slight increase was even observed within the first 72 h). In particular for compound TTZ5 the efficiency was steadily around 6% for the entire duration of the experiment, consistent with the results described above. Interestingly, while the short circuit current density had a small increase at the beginning of the experiment followed by a slow decrease, an opposite pattern was observed for the open circuit voltage, with an initial drop followed by a subsequent recovery; similarly, fill factors increased slightly during the experiment. These results indicate that sensitizers of the thiazolothiazole series are promising candidates for the potential fabrication of large-scale thin-film DSSC modules due to their good performances coupled with high photochemical stability.
 | ||
| Fig. 8 Stability test conducted with 3 μm-thick DSSCs sensitized with dyes TTZ3–5 over the course of 1000 h. Ageing in the dark at 85 °C. | ||
Electrochemical impedance spectroscopy
In the final part of our studies, we analyzed more in detail the charge transfer processes taking place in the cells by means of electrochemical impedance spectroscopy (EIS).49 Measurements for TTZ3–5-containing strip cells (5 μm) were performed in the dark at −0.60 V forward bias over the 0.1 Hz–100 KHz frequency range: the corresponding Nyquist plots (i.e. the plots showing the minus imaginary part of the impedance −Z′′ vs. the real part Z′ when sweeping the frequency) are shown in Fig. 9a, while Bode phase plots (i.e. the plots showing the current/voltage phase shift vs. the frequency) are shown in Fig. 9b. | ||
| Fig. 9 Electrochemical impedance spectra for compounds TTZ3–5 measured in the dark at −0.60 V forward bias: (a) Nyquist plot; (b) Bode phase plot; (c) equivalent circuit. | ||
In the Nyquist plots, due to the use of a liquid electrolyte, two semicircles were observed: the small semicircle at higher frequencies corresponds to charge transfer processes at the Pt–electrolyte interface, while the larger semicircle at lower frequencies derives from the charge transfer processes at the TiO2–dye–electrolyte interface. Several parameters can be obtained by fitting the spectra with a known electrochemical model (see the equivalent circuit in Fig. 9c), namely the series resistance of the cell (Rs), the charge transfer resistance at the TiO2-dye-electrolyte interface (Rrec) and the charge transfer resistance at the counter electrode (RCE). Here, while Rs and RCE were comparable for all dyes (reflecting the similar fabrication conditions and the same materials used in the counter electrode), Rrec values increased in the order TTZ4 (22.0 Ω) < TTZ3 (30.2 Ω) < TTZ5 (37.2 Ω), suggesting that for the latter dye a slower charge recombination was taking place at the photoanode. Considering the Bode phase plots, the frequency of the peaks observed in the middle-frequency domain can be used to evaluate the electron lifetime within the semiconductor (τe),50 providing another indication of the charge recombination rate. In agreement with the previous measurements, we found that electron lifetimes increased in the order TTZ4 (15.1 ms) < TTZ3 (17.5 ms) < TTZ5 (23.2 ms), confirming that electron recombination was more efficiently prevented by the dye with the alkylthio-terminal chains. As recently pointed out in other studies,51 electron lifetime, influenced by factors such as molecular size and dye adsorption behaviour, exerts a strong influence on cell photovoltage. Indeed, also here, the trend in τe values was in good agreement with Voc values measured for the cells and reported in Table 4.
Finally, we also measured EIS spectra at different potentials for all three dyes (see Fig. S13† for the corresponding Nyquist plots). As expected, with a decrease in the bias potential from −0.80 to −0.45 V the interfacial charge recombination process gave rise to a progressively larger semicircle in the mid-frequency region; at high potential, both interfacial charge transfer- and transport resistances within the TiO2 became smaller due to the increased electron density, and a small feature at the lowest applied frequencies became visible as an effect of the diffusion of the I3− ions in the electrolyte.
Conclusions
In this work, five new thiazolo[5,4-d]thiazole-based organic dyes (TTZ3–7) were synthesized and characterized with the aim to use them as sensitizers in thin-layer DSSCs (TiO2 thickness ≤ 6.5 μm). The new compounds displayed broad and intense absorption spectra in the visible region, with impressive molar absorptivities up to 9.67 × 104 M−1 cm−1. Small-scale solar cells built with TTZ3–7, both transparent and opaque, gave good power conversion efficiencies (η up to 7.71%), which in the case of TTZ5 and TTZ7 were clearly superior to those obtained with standard Ru-dye Z907. Larger-scale strip cells (active area: 3.6 cm2), featuring thin films of transparent TiO2 (3–5 μm) and a high stability electrolyte, gave efficiencies in line with those obtained with the smaller devices, with TTZ5 being once again the best sensitizer (η up to 6.35%). Remarkably, in this case employment of CDCA was not necessary, since addition of the co-adsorbent to the sensitizing bath did not lead to any improvement in cell efficiencies compared to the original devices. Even more importantly, all the dyes examined at this stage (TTZ3–5) led to solar cells with very high stability, as shown by a 1000 h storage test at 85 °C, at the end of which efficiencies resulted practically unchanged compared to initial ones.Finally, the charge transfer processes within the cells were investigated by means of electrochemical impedance spectroscopy. These measurements indicated that dye TTZ5 was able to minimize electron recombination from the semiconductor to the oxidized electrolyte more efficiently than the other sensitizers, resulting in a longer charge carrier lifetime and higher Voc. Such data, coupled with the observation reported by Berlinguette et al. that dyes with alkylthio-substituted triphenylamine donor units are more efficiently regenerated compared to their oxygenated or unsubstituted analogs,25 could contribute to explain the superior photovoltaic performances exhibited by dye TTZ5 in comparison with the other sensitizers described in this study.
We are currently trying to expand our library of thiazolothiazole-based sensitizers and develop a synthetic procedure suitable for their large-scale preparation. The dyes will then be used in the fabrication of transparent, thin film DSSC modules to be subjected to even more demanding efficiency and stability measurements. The results of such investigations will be reported in due course.
Experimental
Transparent photoanodes for small-scale DSSCs were prepared by screen-printing a commercial TiO2 paste (Dyesol 18NR-T) on a 8 Ω sq−1 conductive glass substrate (Pilkington), and by sintering the resulting electrodes at 520 °C for 30 minutes. After sintering, the thickness of the semiconductor layer was measured by means of a profiler (Dektak 150, Veeco) and determined to be 5.5 μm. Opaque photoanodes were obtained following an analogous procedure, but using a different commercial titania paste (Dyesol 18NR-AO); their final thickness was 6.5 μm. In both cases, the electrode active area was 0.25 cm2. Counter electrodes were obtained by screen printing a commercial platinum-containing paste (Chimet) on pre-drilled conductive glass plates and by heating at 420 °C for 15 minutes. TiO2 photo-electrodes were sensitized by overnight immersion at rt into the appropriate dye solution (0.1 mM in THF for dyes TTZ3–7, 0.2 mM in EtOH for dye D5, 0.3 mM in EtOH for dye Z907), either in the absence or in the presence of 1 mM chenodeoxycholic acid (CDCA). After sensitization, the anodes were rinsed with EtOH and deionized water, and then dried. A TiO2-sensitized photoanode and a Pt counter electrode were assembled into a sealed sandwich-type cell using a 25 μm hot-melt Surlyn® gasket (Solaronix). A drop of the I−/I3− containing commercial HPE electrolyte solution (Dyesol) was placed on the drilled hole on the back of the counter electrode and was driven into the cell by vacuum backfilling. The hole was finally sealed by using additional sealing film and a small glass cover. Fabrication of strip cells (3.6 cm2 active area) was carried out following the same procedure, except that only transparent photoanodes were prepared, in a thickness of 3 and 5 μm, and a different electrolyte (Dyesol HSE) was used to fill the cells. Sensitization of the photoanodes with standard sensitizer D35 was performed using a 0.3 mM solution of the dye in 1-methoxy-2-propanol.The devices underwent photovoltaic characterization by using a AM 1.5G solar simulator equipped with a Xenon lamp (KHS Solar Constant 1200). The measurements were performed with a power of incoming radiation of 100 mW cm−2. J/V curves were obtained by applying an external bias to the cell and measuring the generated photocurrent with a Keithley model 2400 digital source-meter, under the control of dedicated LabTracer 2.0 software. A black shading mask was used to avoid overestimation of the measured parameters. IPCE spectra were measured with a dedicated apparatus built with the following components: Newport model 70612 Xenon lamp (150 W), Cornerstone 130 1/8 m monochromator and Keithley model 2400 digital source-meter.
Electrochemical impedance spectroscopy (EIS) measurements were performed in the dark using a AUTOLAB 302N potentiostat (Metrohm) equipped with a Nova electrochemical interface system, working at −0.80 V, −0.60 V and −0.45 V forward bias. The spectra were recorded over a frequency range of 10−1 Hz to 105 Hz with an amplitude of 10 mV. Data fitting was carried out using the EC-Lab software (V9.46).
Acknowledgements
The authors thank the “Ente Cassa di Risparmio di Firenze” foundation (“IRIS” project) and MIUR (“Progetto Premiale 2011: Produzione di Energia da Fonti Rinnovabili”) for financial support. A. S. and R. B. thank CINECA and CREA (Colle Val d’Elsa, Italy) for the availability of high performance computing resources and financial support. In addition, the authors wish to thank Dr Francesca De Rossi (C.H.O.S.E., Rome, Italy) for performing the EIS measurements.Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- Dye-sensitized solar cells, ed. K. Kalyanasundaram, EPFL Press, Lausanne, 2010 Search PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- Recent examples: (a) C. Y. Chen, M. Wang, J. Y. Li, N. Pootrakulchote, L. Alibabaei, C. H. Ngoc-le, J. D. Decoppet, J. H. Tsai, C. Grätzel, C. G. Wu, S. M. Zakeeruddin and M. Grätzel, ACS Nano, 2009, 3, 3103–3109 CrossRef CAS PubMed; (b) Y. Numata, S. P. Singh, A. Islam, M. Iwamura, A. Imai, K. Nozaki and L. Han, Adv. Funct. Mater., 2013, 23, 1817–1823 CrossRef CAS.
- L.-L. Li and E. W.-G. Diau, Chem. Soc. Rev., 2013, 42, 291–304 RSC.
- (a) H. J. Snaith, J. Phys. Chem. Lett., 2013, 4, 3623–3630 CrossRef CAS; (b) S. Kazim, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed., 2014, 53, 2812–2825 CrossRef CAS PubMed.
- M. Zhang, Y. Wang, M. Xu, W. Ma, R. Li and P. Wang, Energy Environ. Sci., 2013, 6, 2944–2949 CAS.
- J.-H. Yum, T. W. Holcombe, Y. Kim, K. Rakstys, T. Moehl, J. Teuscher, J. H. Delcamp, M. K. Nazeeruddin and M. Grätzel, Sci. Rep., 2013, 3, 2446 Search PubMed.
- J. Yang, P. Ganesan, J. Teuscher, T. Moehl, Y. J. Kim, C. Yi, P. Comte, K. Pei, T. H. Holcombe, M. K. Nazeeruddin, J. Hua, S. M. Zakeeruddin, H. Tian and M. Grätzel, J. Am. Chem. Soc., 2014, 136, 5722–5730 CrossRef CAS PubMed.
- K. Kakiage, Y. Aoyama, T. Yano, T. Otsuka, T. Kyomen, M. Unno and M. Hanaya, Chem. Commun., 2014, 50, 6379–6381 RSC.
- Y. Ooyama and Y. Harima, ChemPhysChem, 2012, 13, 4032–4080 CrossRef CAS PubMed.
- S. De Sousa, C. Olivier, L. Ducasse, G. Le Bourdon, L. Hirsch and T. Toupance, ChemSusChem, 2013, 6, 993–996 CrossRef CAS PubMed.
- T. Miyasaka, J. Phys. Chem. Lett., 2011, 2, 262–269 CrossRef CAS.
- S. Yoon, S. Tak, J. Kim, Y. Jun, K. Kang and J. Park, Build. Environ., 2011, 46, 1899–1904 CrossRef PubMed.
- H. Zervos, Dye Sensitized Solar Cells (DSSC/DSC) 2013-2023: Technologies, Markets, Players, IDTechEx Report, 2012, can be found at: http://www.idtechex.com/research/reports/dye-sensitized-solar-cells-dssc-dsc-2013-2023-technologies-markets-players-000345.asp Search PubMed.
- S. Ito, T. N. Murakami, P. Comte, P. Liska, C. Grätzel, M. K. Nazeeruddin and M. Grätzel, Thin Solid Films, 2008, 516, 4613–4619 CrossRef CAS PubMed.
- M. L. Parisi, S. Maranghi and R. Basosi, Renewable Sustainable Energy Rev., 2014, 39, 124–138 CrossRef CAS PubMed.
- (a) A. Dessì, G. Barozzino Consiglio, M. Calamante, G. Reginato, A. Mordini, M. Peruzzini, M. Taddei, A. Sinicropi, M. L. Parisi, F. Fabrizi de Biani, R. Basosi, R. Mori, M. Spatola, M. Bruzzi and L. Zani, Eur. J. Org. Chem., 2013, 1916–1926 CrossRef; (b) L. Zani, G. Reginato, A. Mordini, M. Calamante, M. Peruzzini, M. Taddei, A. Sinicropi, M. L. Parisi, F. Fabrizi de Biani, R. Basosi, A. Cavallaro and M. Bruzzi, Tetrahedron Lett., 2013, 54, 3944–3948 CrossRef CAS PubMed.
- W. Zhang, Q. Feng, Z.-S. Wang and G. Zhou, Chem.–Asian J., 2013, 8, 939–946 CrossRef CAS PubMed.
- (a) D. Bevk, L. Marin, L. Lutsen, D. Vanderzande and W. Maes, RSC Adv., 2013, 3, 11418–11431 RSC; (b) L. Zani, M. Calamante, A. Mordini and G. Reginato, in Targets in Heterocyclic Systems, ed. O. A. Attanasi and D. Spinelli, Società Chimica Italiana, Rome, 2014, vol. 17, pp. 87–124 Search PubMed.
- B. G. Kim, K. Chung and J. Kim, Chem.–Eur. J., 2013, 19, 5220–5230 CrossRef CAS PubMed.
- Y. Liang, B. Peng, J. Liang, Z. Tao and J. Chen, Org. Lett., 2010, 12, 1204–1207 CrossRef CAS PubMed.
- A. Dessì, M. Calamante, A. Mordini, M. Peruzzini, A. Sinicropi, R. Basosi, F. Fabrizi de Biani, M. Taddei, D. Colonna, A. Di Carlo, G. Reginato and L. Zani, Chem. Commun., 2014, 50, 13952–13955 RSC.
- (a) A. D. J. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS PubMed; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS; (c) P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- K. C. D. Robson, K. Hu, G. J. Meyer and C. P. Berlinguette, J. Am. Chem. Soc., 2013, 135, 1961–1971 CrossRef CAS PubMed.
- H. Tian, X. Yang, R. Chen, Y. Pan, L. Li, A. Hagfeldt and L. Sun, Chem. Commun., 2007, 3741–3743 RSC.
- T. Yanai, D. Tew and N. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS PubMed.
- B. C. Thompson, Y.-G. Kim, T. D. McCarley and J. R. Reynolds, J. Am. Chem. Soc., 2006, 128, 12714–12725 CrossRef CAS PubMed.
- A. Dessì, M. Calamante, A. Mordini, L. Zani, M. Taddei and G. Reginato, RSC Adv., 2014, 3, 1322–1328 RSC.
- R. Ziessel, A. Nano, E. Heyer, T. Bura and P. Retailleau, Chem.–Eur. J., 2013, 19, 2582–2588 CrossRef CAS PubMed.
- L. Chen, B. Zhang, Y. Cheng, Z. Xie, L. Wang, X. Jing and F. Wang, Adv. Funct. Mater., 2010, 20, 3143–3153 CrossRef CAS.
- (a) C. G. Bates, R. K. Gujadhur and D. Venkataraman, Org. Lett., 2002, 4, 2803–2806 CrossRef CAS PubMed; (b) K. C. D. Robson, B. D. Koivisto and C. P. Berlinguette, Inorg. Chem., 2012, 51, 1501–1507 CrossRef CAS PubMed.
- Y.-H. Seo, W.-H. Lee, J.-H. Park, C. Bae, Y. Hong, J.-W. Park and I.-N. Kang, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 649–658 CrossRef CAS.
- C. Djerassi and C. R. Scholz, J. Am. Chem. Soc., 1948, 70, 417–418 CrossRef CAS.
- A. K. Mohanakrishnan, A. Hucke, M. A. Lyon, M. V. Lakshmikantham and M. P. Cava, Tetrahedron, 1999, 55, 11745–11754 CrossRef CAS.
- (a) J. Tauc, Mater. Res. Bull., 1968, 3, 37–46 CrossRef CAS, application on molecular dyes: (b) C. Coluccini, N. Manfredi, M. M. Salamone, R. Ruffo, M. G. Lobello, F. De Angelis and A. Abbotto, J. Org. Chem., 2012, 77, 7945–7956 CrossRef CAS PubMed.
- Y. J. Chang and T. J. Chow, J. Mater. Chem., 2011, 21, 9523–9531 RSC.
- G. Boschloo and A. Hagfeldt, Acc. Chem. Res., 2009, 42, 1819–1826 CrossRef CAS PubMed.
- Z. Wang, M. Liang, L. Wang, Y. Hao, C. Wang, Z. Sun and S. Xue, Chem. Commun., 2013, 49, 5748–5750 RSC.
- X. Wang, J. Yang, H. Yu, F. Li, L. Fan, W. Sun, Y. Liu, Z. Y. Koh, J. Pan, W.-L. Yim, L. Yan and Q. Wang, Chem. Commun., 2014, 50, 3965–3968 RSC.
- D. P. Hagberg, T. Edvinsson, T. Marinado, G. Boschloo, A. Hagfeldt and L. Sun, Chem. Commun., 2006, 2245–2247 RSC.
- D. P. Hagberg, T. Marinado, K. M. Karlsson, K. Nonomura, P. Qin, G. Boschloo, T. Brinck, A. Hagfeldt and L. Sun, J. Org. Chem., 2007, 72, 9550–9556 CrossRef CAS PubMed.
- L.-P. Heiniger, P. G. O'Brien, N. Soheilnia, Y. Yang, N. P. Kherani, M. Grätzel, G. A. Ozin and N. Tétrault, Adv. Mater., 2013, 25, 5734–5741 CrossRef CAS PubMed.
- P. Wang, S. M. Zakeeruddin, J. E. Moser, M. K. Nazeeruddin, T. Sekiguchi and M. Grätzel, Nat. Mater., 2003, 2, 402–407 CrossRef CAS PubMed.
- L. Schmidt-Mende, J. E. Kroeze, J. R. Durrant, M. K. Nazeeruddin and M. Grätzel, Nano Lett., 2005, 5, 1315–1320 CrossRef CAS.
- D. P. Hagberg, X. Jiang, E. Gabrielsson, M. Linder, T. Marinado, T. Brinck, A. Hagfeldt and L. Sun, J. Mater. Chem., 2009, 19, 7232–7238 RSC.
- Dye D35 is commercially available from Dyenamo AB (Sweden). For a list of references regarding applications of such dye, see: http://www.dyenamo.se/dyenamo_dyes.php.
- Y. Huang, S. Dai, S. Chen, C. Zhang, Y. Sui, S. Xiao and L. Hu, Appl. Phys. Lett., 2009, 95, 243503 CrossRef PubMed.
- F. Fabregat-Santiago, G. Garcia-Belmonte, I. Mora-Seró and J. Bisquert, Phys. Chem. Chem. Phys., 2011, 13, 9083–9118 RSC.
- Q. Li, L. Lu, C. Zhong, J. Huang, Q. Huang, J. Shi, X. Jin, T. Peng, J. Qin and Z. Li, Chem.–Eur. J., 2009, 15, 9664–9668 CrossRef CAS PubMed.
- L. Yang, Z. Zheng, Y. Li, W. Wu, H. Tian and Z. Wang, Chem. Commun., 2015, 51, 4842–4845 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional computational data, general synthetic remarks, synthesis and characterization of all new compounds, additional photoelectrochemical measurements, copies of the NMR spectra of compounds 7, 15, 19–20, 23, TTZ6 and 7. See DOI: 10.1039/c5ra03530a |
| This journal is © The Royal Society of Chemistry 2015 |