High concentration DNA solubility in bio-ionic liquids with long-lasting chemical and structural stability at room temperature†
Abstract
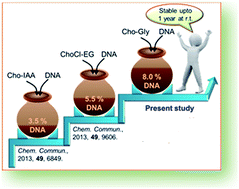
DNA (Salmon testes) was solubilized in two bio-based ionic liquids (bio-ILs) namely choline-pyruvate (Cho-Pyr) and choline-glycolate (Cho-Gly) up to 2.0 and 8.0 wt% respectively. The solubilized DNA was found to maintain its chemical and structural stability for up to one year when stored at room temperature (25 °C). Excessive H-bonding along with electrostatic interactions between the DNA and the ILs, as observed in the isothermal titration calorimetry (ITC) studies, was found to be the major reason behind the high concentration of DNA dissolution in Cho-Gly and its long term stability. Measurements carried out on a UV-Vis spectrophotometer, FT-IR, circular dichroism (CD) spectrometer, agarose-gel electrophoresis and PCR amplification of recovered DNA from the ILs solutions confirmed the chemical and structural integrity of DNA during dissolution.


 Please wait while we load your content...
Please wait while we load your content...