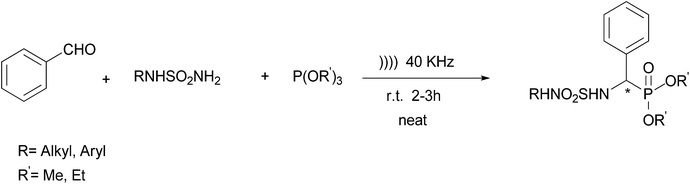
A one-pot three-component synthesis of novel α-sulfamidophosphonates under ultrasound irradiation and catalyst-free conditions†
Billel Belhania,
Malika Berredjem*a,
Marc Le Borgneb,
Zouhair Bouazizb,
Jacques Lebretonc and
Nour-Eddine Aoufa
aLaboratory of Applied Organic Chemistry, Synthesis of Biomolecules and Molecular Modelling Group, Sciences Faculty, Chemistry Department, BadjiMokhtar – Annaba University, Box 12, 23000 Annaba, Algeria. E-mail: malika.berredjem@univ-annaba.org; mberredjem@yahoo.fr
bUniversité de Lyon, Université Lyon 1, Faculté de Pharmacie e ISPB, EA 4446 Biomolécules Cancer et Chimiorésistances, SFR Santé Lyon-Est, CNRS UMS3453 e INSERM US7, 8 Avenue Rockefeller, F-69373 Lyon Cedex 8, France
cUniversité de Nantes, UMR CNRS 6230, Chimie Et Interdisciplinarité: Synthèse, Analyse, Modélisation (CEISAM), UFR des Sciences et des Techniques, 2, rue de la Houssinière, BP 92208, 44322 Nantes Cedex 3, France
First published on 21st April 2015
Abstract
An efficient and convenient one-pot synthesis of novel α-sulfamidophosphonates is described via a three-component reaction. This reaction was carried out through a three component condensation reaction of sulfonamide, an aromatic aldehyde and triethylphosphite under conventional/ultrasonic techniques, catalyst-free and solvent-free conditions. This methodology was established with many advantages, including mild reaction conditions, short reaction times, good yields, simple work-up procedures, and environmental friendliness.
Introduction
α-Aminophosphonate have found a wide range of applications in medicinal chemistry, and they are considered to be enzyme inhibitors,1 antibiotics,2 pharmacological agents,3 and peptidomimetics.4 But surprisingly α-sulfamidophosphonates, new sulfonamide derivatives, to the best of our knowledge, have not been described. In the literature novel phosphonates containing a sulfonamide moiety have been described and have interesting biological properties. They act as potent inhibitors of protein tyrosine phosphatase 1B and HIV protease inhibitors.5,6A number of synthetic methods for the construction of α-aminophosphonates derivatives have been reported. Generally, these methods could be performed in the catalysis of Bronsted or Lewis acids like BF3/Et2O, ZnCl2, MgBr2, SnCl4, etc.7–9 However, in spite of their potential utility, these methods typically suffer from more disadvantages such as high cost of the catalyst, use of a stoichiometric amount of reagent and occurrence of several side reactions. The first multicomponent synthesis of α-aminophosphonates has been achieved by Kabachnik–Fields10–15 in the presence of different catalyst.
Multicomponent reactions are efficient and effective methods particularly well suited for diversity-oriented synthesis, they can be defined as convergent chemical processes where three or more reagents react together via a one-pot procedure in such a way that the final product retains significant portions of all starting materials.16 Such reactions present remarkable advantages for library synthesis aimed at carrying out structure–activity relationship (SAR) studies of drug-like compounds, in a single procedural step such as; high degree of atom economy, reduction in reaction steps and the number of workup, reduction in energy consumption.17 This methodological approach has now found various applications in synthesis of pharmaceutically active compounds, and marine alkaloids and derivatives.18
Many different process parameters such as temperature, pressure, solvent, catalyst type, Microwave and ultrasonic irradiations, and other factors can be utilized to modulate the selectivity of synthetic transformations.
One of the powerful tools used to connect economic features with the green concerns is performing organic reactions under ultrasound irradiation and solvent-free conditions.19–21 This powerful technique became extremely efficient and attractive in synthetic organic chemistry, and is able to activate many reactions due to cavitational collapse. Ultrasound irradiation provides higher yields and selectivities, shorter reaction times and milder reaction conditions, nontoxic, environmentally friendly solvent, in a one-step reaction, without isolation of any intermediate thus reducing time, saving money, energy and raw materials. In this research, we report a highly efficient one-pot, three component condensation reaction for the synthesis α-sulfamidophosphonate derivatives 2 under ultrasound irradiation, catalyst and solvent-free conditions in high yields.
Results and discussion
Initially, the sulfonamides presented here were obtained in three steps from a simple and efficient methodology described by our group.22–26Herein we studied the one-pot synthesis of α-sulfamidophosphonates under solvent-free reaction conditions using ultrasound. Initially, benzaldehyde was reacted with structurally diverse sulfonamide and triethylphosphite in the absence of any solvent and any catalyst after 2–3 hours, the reaction was completed with an excellent yield (Scheme 1).
To find the effect of ultrasound, the same reaction was carried out under the same conditions in the absence of ultrasound irradiation. No reaction occurs after 5 h working time, this shows the essential role of ultrasound irradiation. This excellent result encourages us to extend this study to various structurally amines. To optimize our protocol, we also applied our reaction conditions to a number of primary and secondary aromatic and aliphatic (cyclic and acyclic) amines. The results of these studies are presented in Table 1, (entries 2a–q).
| Entry | Amine | Compound | Time (hours) | Yield (%) | M.p. (°C) |
|---|---|---|---|---|---|
| a Conditions: aldehyde (1 mmol), sulfonamide (1 mmol), triethylphosphite (1 mmol), 40 kHz, r.t. | |||||
| 2a |  |
 |
2 | 95 | 152–154 |
| 2b | 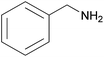 |
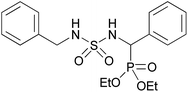 |
2 | 89 | 143–145 |
| 2c | 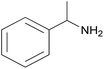 |
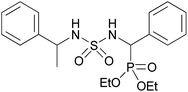 |
2.5 | 90 | 138–140 |
| 2d | 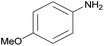 |
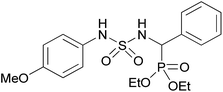 |
3 | 87 | — |
| 2e | 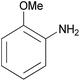 |
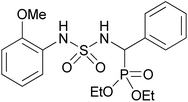 |
3 | 85 | 145–147 |
| 2f | 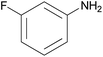 |
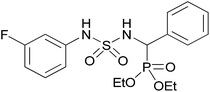 |
1.5 | 92 | 109–111 |
| 2g | 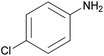 |
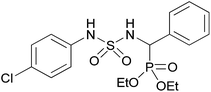 |
2 | 91 | 116–118 |
| 2h | 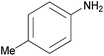 |
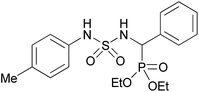 |
2 | 84 | — |
| 2i | 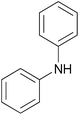 |
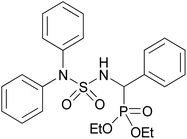 |
3 | 70 | 156–158 |
| 2j | 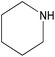 |
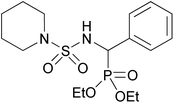 |
3 | 65 | — |
| 2k | 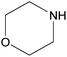 |
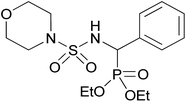 |
3 | 75 | — |
| 4l | 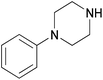 |
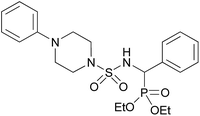 |
3 | 80 | 147–149 |
| 2m | 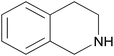 |
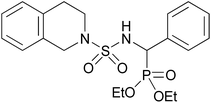 |
3 | 73 | — |
| 2n | 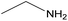 |
 |
1.5 | 82 | 131–133 |
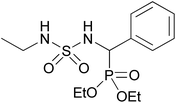
| 2o |  |
 |
2 | 88 | 136–138 |
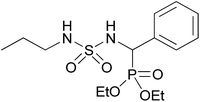
| 2p |  |
 |
2.5 | 86 | 135–137 |
| 2q | 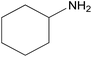 |
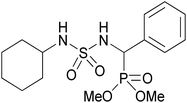 |
1.5 | 94 | 137–139 |
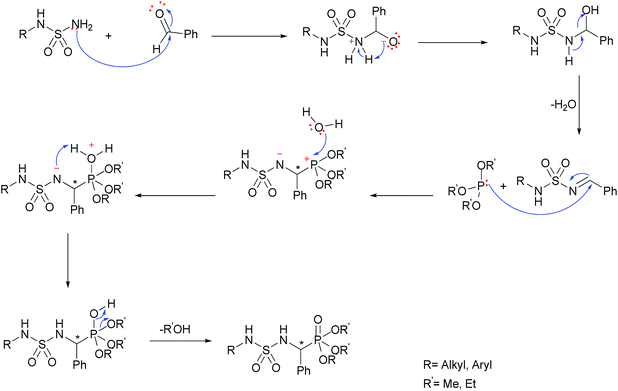
Mechanistic proposal
The presented results demonstrate the specific ultrasonic effect on multicomponent reactions giving pure product with quantitative yields in a few minutes. The ultrasonic energy applying to the reaction generates the acoustic cavitation mechanical effect when sonic waves propagate through the medium. In solids, both longitudinal and transverse waves can be transmitted whereas in liquids only longitudinal waves can be transmitted. Vibrations of molecules generate compressions and rarefactions which give rise to the phenomenon of bubble formation and collapse in the reaction mixture [sulfonamide, amine and triethylphosphite] and facilitate the nucleophilic attack of the amino functional on the carbonyl group. During cavitation, the chemical bonds break, and H2O and EtOH were eliminated to afford the α-sulfamidophosphonate according to the mechanism below (Scheme 2).Experimental
Materials
The chemicals used in this work were obtained from Fluka and Merck Chemical Company and were used without purification.Apparatus
Melting points were measured in open capillary tubes on an electro thermal apparatus and uncorrected. Mass spectra were recorded on a Shimadzu QP 1100 Ex mass spectrometer operating at an ionization potential of 70 eV. IR spectra were recorded as KBr pellets on a Perkin Elmer 781 spectrophotometer and an Impact 400 Nicolet FT-IR spectrophotometer. 1H NMR, 13C NMR and 31P NMR spectra were recorded in DMSO-d6 or CDCl3 solvents on a 250, 300 or 400 MHz Bruker spectrometer with tetramethylsilane as internal reference.Ultrasound assisted reactions were carried out using a FUNGILAB ultrasonic bath with a frequency of 40 kHz and a nominal power of 250 W. The reactions were carried out in an open glass tube (diameter: 25 mm; thickness: 1 mm; volume: 20 mL) at room temperature. All reactions were monitored by thin layer chromatography (TLC) on silica Merck 60 F254 percolated aluminum plates.
General procedure
In a 10 mL round bottom flask taken a mixture of aldehyde (1 mmol) and sulfonamide (1 mmol) at room temperature and then triethylphosphite (1 mmol) was added. Then reaction mixture was subjected to the ultrasonication for appropriate time. After completion of the reaction, as indicated by TLC, silica gel; dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (9
methanol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), a (4
1), a (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture of diethyl ether and n-hexane was added and the mixture was cooled to 6 °C overnight. The product was finally filtered and dried.
1) mixture of diethyl ether and n-hexane was added and the mixture was cooled to 6 °C overnight. The product was finally filtered and dried.
Conclusions
In conclusion, we have developed a facile and efficient one-pot, three-component synthesis of α-sulfamidophosphonates under ultrasound irradiation, solvent- and catalyst-free conditions at room temperature in high yields. This reaction system provides a novel method for the synthesis of biologically important α-sulfamidophosphonates. The method offers several advantages including high yield of products and easy experimental work-up procedure.Acknowledgements
This work was supported financially by The General Directorate for Scientific Research and Technological Development (DG-RSDT), Algerian Ministry of Scientific Research, Applied Organic Laboratory (FNR 2000).Notes and references
- (a) M. C. Allen, W. Fuhrer, B. Yuck, R. Wade and J. M. Wood, J. Med. Chem., 1989, 32, 1652 CrossRef CAS; (b) E. W. Logusch, D. M. Walker, J. F. McDonald, G. C. Leo and J. E. Franz, J. Org. Chem., 1988, 53, 4069 CrossRef CAS; (c) P. P. Giannousis and P. A. Bartlett, J. Med. Chem., 1987, 30, 1603 CrossRef CAS.
- M. V. N. Reddy, S. Annar, A. Balakrishna, G. C. S. Reddy and C. S. Reddy, Org. Commun., 2010, 3, 39 CAS.
- F. R. Atherton, C. H. Hassall and R. W. Lambert, J. Med. Chem., 1986, 29, 29 CrossRef CAS.
- P. Kafarski and B. Lejczak, Phosphorus, Sulfur Silicon Relat. Elem., 1991, 63, 1993 CrossRef.
- T. Cihlar, G. X. He, X. Liu, J. M. Chen, M. Hatada, S. Swaminathan, M. J. McDermott, Z. Y. Yang, A. S. Mulato, X. Chen, S. A. Leavitt, K. M. Stray and W. A. Lee, J. Mol. Biol., 2006, 363, 635 CrossRef CAS PubMed.
- C. P. Holmes, X. Li, Y. Pan, C. Xu, A. Bhandari, C. M. Moody, J. A. Miguel, S. W. Ferla, M. N. De Francisco, B. T. Frederick, S. Zhou, N. Macher, L. Jang, J. D. Irvine and J. R. Grove, Bioorg. Med. Chem. Lett., 2005, 15, 4336 CrossRef CAS PubMed.
- K. A. Petov, V. A. Chauzov and T. S. Erkhina, Usp. Khim., 1974, 43, 2045 (Chem. Abstr., 1975, 82, 449) Search PubMed.
- S. Laschat and H. Kunz, Synthesis, 1992, 90 CrossRef CAS.
- J. Zou, Pol. J. Chem., 1981, 55, 643 Search PubMed.
- (a) M. I. Kabachnik and T. Y. Medved, Dokl. Akad. Nauk SSSR, 1952, 83, 689 CAS; (b) A. Heydari, A. Karimian and J. Ipaktschi, Tetrahedron Lett., 1998, 39, 6729 CrossRef CAS.
- (a) N. Azizi and M. R. Saidi, Eur. J. Org. Chem., 2003, 11, 4630 CrossRef PubMed; (b) S. Chandrasekhar, S. J. Prakash, V. Jagadeshwar and C. Narsihmulu, Tetrahedron Lett., 2001, 42, 5561 CrossRef CAS.
- B. C. Ranu, A. Hajra and U. Jana, Org. Lett., 1999, 1, 1141 CrossRef CAS.
- K. Manabe and S. Kobayashi, Chem. Commun., 2000, 669 RSC.
- B. Kaboudin and A. Rahmani, Synthesis, 2003, 2705 CrossRef CAS PubMed.
- S. Lee, J. H. Park, J. Kang and J. K. Lee, Chem. Commun., 2001, 1698 RSC.
- (a) I. Ugi and C. Steinbrückner, Chem. Ber., 1961, 94, 734 CrossRef CAS PubMed; (b) I. Ugi, Pure Appl. Chem., 2001, 73, 187 CrossRef CAS; (c) A. Domling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (d) D. M. D'Souza and T. Mueller, J. Chem. Soc. Rev., 2007, 36, 3169 Search PubMed; (e) C. C. A. Cariou, G. J. Clarkson and M. Shipman, J. Org. Chem., 2008, 73, 9762 CrossRef CAS PubMed; (f) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (g) L. Weber, K. Illegen and M. Almstetter, Synlett, 1999, 8, 366 CrossRef PubMed; (h) G. H. Posner, Chem. Rev., 1986, 86, 831 CrossRef CAS; (i) R. V. A. Orru and M. de Greef, Synthesis, 2003, 10, 1471 CrossRef PubMed; (j) E. N. da Silva, Res. J. Chem. Environ., 2007, 11, 90 Search PubMed.
- J. Zhu and H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, Germany, 2005 Search PubMed.
- B. B. Tour and D. G. Hall, Chem. Rev., 2009, 109, 4439 CrossRef PubMed.
- G. Gravotto and P. Cintas, Chem. Soc. Rev., 2006, 35, 180 RSC.
- (a) G. A. Dilbeck, L. Field, A. A. Gallo and R. J. Gargiulo, J. Org. Chem., 1978, 43, 4593 CrossRef CAS; (b) G. S. Lv, F. J. Duan, J. C. Ding, T. X. Cheng, W. X. Gao, J. X. Cheng and H. Y. Wu, J. Chem. Sci., 2012, 124, 1057 CrossRef CAS PubMed; (c) W. X. Guo, H. L. Jin, J. X. Chen, F. Chen, J. C. Ding and H. Y. Wu, J. Braz. Chem. Soc., 2009, 20, 1674 CrossRef CAS PubMed; (d) B. Belhani, A. Bouzina, M. Berredjem and N. E. Aouf, Monatsh. Chem., 2015 DOI:10.1007/s00706-015-1461-4.
- A. Amira, H. K'tir, M. Berredjem and N. E. Aouf, Monatsh. Chem., 2014, 145, 509 CrossRef CAS PubMed.
- W. Boufas, N. Dupont, M. Berredjem, K. Berrezag, I. Becheker, H. Berredjem and N. E. Aouf, J. Mol. Struct., 2014, 1074, 180 CrossRef CAS PubMed.
- M. Berredjem, R. Bouasla, N. E. Aouf and C. Barbey, X-Ray Struct. Anal. Online, 2010, 26, 13 CrossRef CAS.
- C. Bougheloum, C. Barbey, M. Berredjem, A. Messalhi and N. Dupont, J. Mol. Struct., 2013, 10, 41 Search PubMed.
- C. Barbey, R. Bouasla, M. Berredjem, N. Dupont, P. Retailleau, N. E. Aouf and M. Lecouvey, Tetrahedron, 2012, 68, 9125 CrossRef CAS PubMed.
- M. Berredjem, S. Ait Kaki and N. E. Aouf, Arabian J. Chem., 2014 DOI:10.1016/j.arabjc.2013.01.016.
Footnote |
| † Electronic supplementary information (ESI) available: Spectral data for the synthesis of novel α-sulfamidophosphonate prepared in this work. See DOI: 10.1039/c5ra03473f |
| This journal is © The Royal Society of Chemistry 2015 |