Preparation and properties of stretchable and tough alginate/polyacrylamide hollow capsules†
Ming-Lu Zhu,
Yan-Li Li,
Zi-Mou Zhang and
Yong Jiang*
School of Chemistry and Chemical Engineering, Southeast University, Jiangning, Nanjing, Jiangsu 211189, P. R. China. E-mail: yj@seu.edu.cn; Web: http://jianglab.net Tel: +86-139-139-931-09
First published on 25th March 2015
Abstract
Encapsulation technology has important applications in drug delivery, catalysis, sensing and photonics. In this paper, a stretchable and tough hollow capsule was synthesized using alginate and polyacrylamide (PAAm) as the shell material. Calcium carbonate (CaCO3) microspheres were first chosen as sacrificial templates for the covalent crosslinking of the PAAm network at their surface. Then, they were decomposed using acid to form a hollow capsule, and the released Ca2+ ions were used for the ionic crosslinking of the alginate network. The influence of the density of CaCO3 microspheres on the internal structure of the capsules was explored and the ball loading ball structure was observed by scanning electron microscopy and fluorescence microscopy. The mechanical strength of the prepared capsules was studied by both tensile/compressive testing and an osmotic pressure method, and the results showed that the volume of capsules could be expanded at least 27 times their original sizes without breakage. The release behaviours of the model drug BSA-FITC for three capsules with different crosslinking densities were studied, and the results showed that drug loading capacity and water absorption ratio were proportional to the number of CaCO3 microspheres used during the preparation.
Introduction
Encapsulation technology has important applications in many fields such as drug delivery, catalysis, sensing and photonics.1–4 The shell materials of typical capsules are made of natural or synthetic polymeric materials or inorganic compounds. Normally, capsules were prepared using chemical reactions, phase separation and mechanical physical methods.5–7 With the continuous development of the research and application of capsules, new encapsulation technology is constantly being developed.8,9Mechanical intensity and elasticity are two important properties for the applications of general capsules. However, it is difficult to make capsule with both the properties at the same time. The capsules that are prepared by physical methods show poor intensity. As a result, the polyanion/polycation that forms the shells of capsules will burst when the swelling pressure exceeds a certain value when the alginate gel capsules are in water.10 The main cause of an alginate/polycation capsule breakage under physiological conditions is probably the osmotic swelling of the alginate owing to the Donnan equilibrium set up by the negative charges of the carboxyl groups not involved in cooperative binding of counter ions in drug release.11
Intense efforts have been devoted in order to synthesize capsules with improved mechanical intensity and elasticity, i.e., stretchable and tough capsules. Composite multiple networks have proven to be ideal solutions for preparing hydrogels with improved intensity and elasticity,12–17 which can also be adapted to make capsules. For example, capsules could be made stretchable and tough by introducing energy dissipating parts. Gong et al. synthesized a double-network membrane with a fracture energy of ∼1000 J m−2, which contained two networks: one with short chains and other with long chains.18 However, permanent damage of the short chains limited its reuse. Soon afterwards, the sacrificial covalent bonds were replaced by non-covalent bonds, and a recoverable energy-dissipating network was achieved.19,20 Sun et al. synthesized a highly stretchable and tough composite hydrogel, which contained three types of networks:21 ionic crosslinks formed at the G blocks of different polymer chains through Ca2+ in an alginate gel, covalent crosslinks at the polymer chains in a polyacrylamide (PAAm) gel, as well as the intertwined and joined crosslinks between the two types of polymer networks by covalent crosslinks between amine groups on PAAm chains and carboxyl groups on alginate. The hydrogel can be stretched up to more than 20 times its original length without rupture.
In this study, an alginate/polyacrylamide system was chosen as the shell material for making stretchable and tough hollow capsules. Calcium carbonate (CaCO3) microspheres were chosen as sacrificial templates, and a suspension and core–shell polymerization method was adopted using a water-in-oil system. The influence of the density of CaCO3 microspheres on the internal structure of the capsules and the mechanical strength of capsule were explored in detail. The release behaviours of BSA-FITC as model drug were also investigated.
Experimental section
Materials
Sodium alginate, acrylamide (AAm), N-methylene bisacrylamide (MBA), N,N,N′,N′-tetramethyl ethylene diamine (TEMED), ammonium persulfate (APS), span 80 (HLB = 4.3) and tween 80 (HLB = 15.0) were purchased from Aladdin (Shanghai, China). Fluorescein isothiocyanate labelled bovine serum albumin (BSA-FITC), bovine serum albumin (BSA) and dextran (MW = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800) were obtained from Sangon Biotech (Shanghai, China). Anhydrous calcium chloride (CaCl2) and sodium carbonate (Na2CO3) were purchased from Sino Pharm Chemical Reagent Co., Ltd. (Shanghai, China). Deionized water was prepared by a secondary distillation technique using a doubly distilled water device (YMNL, Nanjing, China). All the chemicals were analytically pure.
800) were obtained from Sangon Biotech (Shanghai, China). Anhydrous calcium chloride (CaCl2) and sodium carbonate (Na2CO3) were purchased from Sino Pharm Chemical Reagent Co., Ltd. (Shanghai, China). Deionized water was prepared by a secondary distillation technique using a doubly distilled water device (YMNL, Nanjing, China). All the chemicals were analytically pure.
Preparation of CaCO3 microspheres
CaCO3 microspheres were prepared using the method previously reported.22,23 In short, 160 ml Na2CO3 solution (0.33 M), 160 ml CaCl2 solution (0.33 M) and 40 ml ethanol were rapidly mixed together with vigorous stirring, and then incubated for 30 s. Then, the resultant CaCO3 microspheres were extracted by Buchner funnel, washed twice with water and third time with ethanol, and dried at 40 °C in a vacuum drying oven.Preparation of “ball-load-ball” hollow capsules
0.05 g alginate was dissolved in 5 ml distilled water, followed by the addition of CaCO3 microspheres with 16 wt% of alginate. The mixture was sonicated for 3 min and incubated for 30 min to get a stable suspension. Then, 0.4 g AAm, 2.4 mg MBA and 2 mg APS were added to the above suspension to form a water phase. At the same time, 2 ml span 80 and 0.5 ml tween 80 were dissolved in 20 ml n-heptane to make an oil phase. The water phase was added to the oil phase and the mixture was stirred at 1500 r/min for 15 min to get a stable emulsion. Finally, 1 mg TEMED was added to the solution as a catalyst. After 5 h of polymerization, 4 ml acetic acid was added to decompose CaCO3, and alginate was crosslinked by the released Ca2+ for a period of 12 h of incubation. Then, the mixture was transferred to a funnel, allowed to stand for 5 h, taking the lower sediment and filtered by Buchner funnel with 0.2 μm pore size. The size of the prepared capsules could be adjusted by adjusting the stirring speed.Fourier transform infrared (FTIR) spectroscopy
The structures of alginate, PAAm and the shell of the alginate and AAm capsules were analysed using an FTIR Spectrometer (Nicolet 6700). Capsules were freeze dried before FTIR measurement.Scanning electron microscopy (SEM) measurement
Both the surface and the internal morphology of the capsules were observed by SEM (Hitachi S-3400-II, 10 kV). The freeze dried capsules were dissected using a scalpel before imaging and a mica sheet was used as a substrate to support the capsule hemispheres.Fluorescence microscope (FM) measurement
The internal structure of the capsules was investigated by FM [Lumencor SOLA]. Capsules loaded with BSA-FITC were prepared by immersing them in a 0.5 mg ml−1 BSA-FITC aqueous solution for 2 h, and then washing with water and centrifugally separating three times.24 Consequently, the BSA-FITC loaded capsules were measured by FM. Excitation and emission wavelengths were 488 and 515 nm, respectively.Measurement of capsule size distribution
In order to study the particular characteristics of the capsules, the size distributions of the capsules were measured by a laser particle size instrument (British Ma Wener). About 200–500 capsules were randomly selected and measured.Measurement of mechanical strength
The tensile and compressive strengths of the alginate/PAAm capsules and pure alginate capsules as a control were measured and compared in detail. For tensile testing, a capsule was fixed in the micro-platform and stretched at a force of 5 N. A microscope was used to record the size changes of the capsule. A Texture Analyzer (Model TA.XT2i, England) was used for compressive testing. Pressure was applied with a compression speed of 0.1 g s−1 and the force was recorded when ten capsules were broken. For each measurement, the test was repeated at least five times and the average value and standard deviation were calculated.Mechanical testing by an osmotic pressure method
First, 200 capsules were randomly chosen and immersed in saturated dextran solution for 12 h. Second, the capsules were washed and centrifugally separated three times with distilled water; then, the saturated capsules were placed in water for two days. Because the concentration of dextran inside the capsule was considerably higher than that outside of the capsule, water permeated into the capsule and the capsule swelled. The size distributions before and after inflation were measured by a laser particle size instrument (Mastersizer 2000). Moreover, the surface morphology of the expanded capsules was studied by optical microscopy. In this experiment, dextran can be substituted by other water soluble polymers that cannot easily penetrated from the capsules.Water absorption of capsules
In order to study the relationship between water absorption and the internal structure of the capsule, three types of capsules were prepared using different densities of CaCO3 microspheres. The samples with known initial weight were first immersed in a dextran solution for 12 h, and then centrifugally filtered three times. After that, the samples were immersed in water for one day. Filter paper was used to remove surface moisture, and the weights of the saturated capsules were determined by electronic balance. The water absorption ratios of three samples were calculated using the following eqn (1).
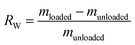 | (1) |
Drug loading and release capacity
Protein release behaviours of loaded capsules were researched by Ultraviolet spectrophotometer. Three types of capsules with different internal volumes were prepared. Then, these capsules were incubated in a BSA solution (2%) for 2 hours, and then immersed into tubes with 2 ml water. 50 μl supernatant fluid was extracted every three hours from the centrifuged solution. The absorption intensities of the supernatant fluids were measured using a UV 2450 at 280 nm. The concentration of the released BSA was acquired, according to its absorption, using the Beer–Lambert's Law.25Results and discussion
Fig. 1 illustrates the strategy for making stretchable and tough hollow microgel capsules. First, CaCO3, AAm and alginate were mixed together in deionized water to form a water phase. SEM images of CaCO3 templates are shown in Fig. S1 (Please see ESI†) for details. Because the surface of the CaCO3 microspheres was positively charged, part of the alginate, which was negatively charged, was absorbed on their surface. Then, the mixture was added into a prepared oil phase to form a water-in-oil system and inverse suspension polymerization was adopted for the preparation of a PAAm crosslinking network.26 Because AAm monomers and CaCO3 microspheres were gathered in aqueous dispersion, polymerization took place inside the aqueous dispersion while between the interfaces of CaCO3 microspheres. After polymerization, the PAAm microgels were formed, including a large number of uncrosslinked alginate and CaCO3 microspheres. When CaCO3 was dissolved using acetic acid, Ca2+ was released and used to crosslink the alginate. Finally, the microgels turned into hollow capsules with multiple cells.Herein, CaCO3 microspheres played two important roles during the preparation of the alginate/PAAm capsules. First, they worked as sacrificial templates for the covalent crosslinking of PAAm networks on their surface. Second, when they were decomposed by acid, the released Ca2+ was used for the ionic crosslinking of the alginate network.
The prepared capsules were measured by FTIR and the results are shown in Fig. S2 (ESI†). A new peak at 1383 cm−1 for the C–N stretching of secondary amides was found comparing the spectrum of the capsule with that of alginate and PAAm. Furthermore, the intensity of primary amide peaks (1636 and 1460 cm−1) and the NH2 in-plane rocking peak (1124 cm−1), as well as the intensities of the O–H stretching peak (3450 cm−1) and symmetric C–O stretching (1090 cm−1), were decreased. All these results indicate new bonds were formed between the –NH2 groups of PAAm and the carboxyl groups of alginate.
The surface morphology and internal structures of the capsules were observed by SEM and FM. As shown in Fig. 2A, the capsules appear to be round balls with rough surfaces. The size was quite uniform with an average diameter of about 380 μm. Although rough porous surfaces existed in such structures, the capsules might still easily maintain a spherical structure without collapsing and fracturing. A capsule was freeze dried and cut in order to observe the interior morphology. The image in Fig. 2B shows the multilayer and multicellular structures of the internal capsule. Moreover, the thickness of the interior walls was almost uniform. A good deal of small CaCO3 microspheres could be observed in the interior wall of the hollow capsule in Fig. 2C. These CaCO3 microspheres have a diameter of about 5 μm. This ball-loading-ball structure in the capsule is similar to that fabricated by water-in-oil techniques in previous studies.27,28 The cavities inside the capsule could be used to load the model drug BSA-FITC. Fig. 2D is a FM image of hollow capsules loaded with BSA-FITC.
The mechanical properties and loading capacity of capsules are the two key factors that influence applications. Mechanical strength not only influences the stability of the capsule during the preparation process, but also decides whether the shell could maintain its initial integrity during transportation and application. The mechanical strength could be detected by strength characterization of the shell of capsules. According to previous research,19 the synthesized alginate/PAAm hydrogels (containing 90% water) using same methods could be stretched beyond 20 times their initial length and have fracture energies of 9000 J m−2. Even for samples containing notches, a stretch of 17 is demonstrated. In this paper, we prepared the hollow capsule using a similar protocol, thus the prepared capsules should be stretchable and tough.
Fig. 3 shows the tensile test images of two types of capsules. Fig. 3A was an original alginate/PAAm capsule. After stretching, the size of the capsule turned into about 4 times longer without breakage, as shown in Fig. 3B. The original size of alginate/PAAm capsules was 323 ± 20 μm. Moreover, the size turned to 1150 ± 113 μm after stretching, which is about 3.6 times longer than its original size on average. Furthermore, the stretched capsule could restore its original spherical shape when the pulling force was released. Fig. S3† demonstrates the recovery process of a stretched alginate/PAAm capsule. At the same time, pure alginate capsules were prepared and stretched as controls. Fig. 3C and D show the optical images of an original capsule and the same capsule after tensile testing. The original size of alginate capsules increased from 387 ± 18 μm to 480 ± 35 μm after stretching. The capsule was only able to be stretched 1.2 times longer before breakage.
Table 1 shows the compressive testing of the two types of capsules. The critical crushing pressure of the alginate/PAAm capsules was 7.9 ± 0.7 N, which was about 6 times higher than that of pure alginate capsules. The alginate/PAAm capsules were considerably tougher than alginate capsules because the interpenetrating structure of alginate and PAAm network in alginate/PAAm capsules made the pressure dissipate easily.
| Diameter (μm) | Pressure (N) | |
|---|---|---|
| Alginate/PAAm capsules | 391 ± 62 | 7.9 ± 0.7 |
| Alginate capsules as control | 350 ± 20 | 1.4 ± 1.1 |
Swelling behaviour is a common phenomenon of capsules during the drug release process, especially in the application of the human body, where the body fluids and blood are the complex. Only capsules with high strength and toughness could avoid breakage. Herein, swelling experiments of capsules after absorbing water was surveyed. The size distribution of the capsules before and after swelling was measured by a laser particle size analyser. As shown in Fig. 4A and C, the average diameter of the initial capsules was 391 ± 62 μm. Then, the capsules were immersed in a saturated dextran solution for 12 h, and then in pure water for two days. Capsules absorbed water and swelled slowly due to the high osmotic pressure. Fig. 4B and D shows the similar capsules, as shown in Fig. 4A, but the size was about 1184 ± 65 μm. The diameter of the expanded capsules was about 3 times larger than its original size without breakage. With the three times increased diameter, volume would expand to about 27 times in theory, which indicates, to a certain extent, that the prepared capsules were stretchable and tough. Furthermore, alginate/PAAm capsules have the ability of loading considerably more drug than general ones. As Fig. 4B shows, after expanded, the shape of the capsule turned slightly oval and the film of the capsule became thinner and smoother, but no ruptures were found on the surface. This indicates that the capsule might be expanded further; however, it is disappointing that the expansion at the breaking point could not be measured by this osmotic pressure method. Thus, we did not know the maximum value of the expansion.
It was found from the above testing that alginate/PAAm capsules were stretchable and tough because they consist of three types of crosslinking networks: covalent crosslinks of PAAm networks through polymerization of AAm by MBA, ion crosslinks of alginate networks after Ca2+ crosslinking the G blocks on different chains, and an alginate/PAAm hybrid network, in which the two types of polymer network are intertwined, and joined by both covalent crosslinks and the hydrogen bonds between the –COOH of alginate and the –CONH2 of AAm. When the capsules were swelled, bonds between alginate and Ca2+ ruptured to protect the covalent bonds from damaging, while the PAAm network could preserve the basic shape of the capsule. Then, the ionic bonds could be restored when the expansion forces disappeared. The composite network of alginate/PAAm capsules made repeated use possible.
One thing that needs to be clarified is that the original size of the capsule that we demonstrate here was around 400 μm, which is considerably larger than the typical capsule for drug release. We present this big capsule just because it was easier for us to measure the mechanical properties.
The ratio of water absorption of capsules is an important factor that directly affects the application because the ratio of water absorption might be proportional to the quantity of drug that could be loaded. Herein, three different types of capsules were prepared using different weight ratios of CaCO3/alginate by controlling the number of CaCO3 microspheres. After the capsules were freeze dried, they were immediately cut into hemispheres, and the cross section of the capsules was measured by SEM to observe the internal morphology. The results in Fig. 5A to C show that the capsules gradually evolved from hollow structures into solid gel beads. When the weight ratio of CaCO3/alginate was 16% in Fig. 5A, the capsule had multiple cavities and the capsule shell was thin. Moreover, when the usage of CaCO3 microspheres decreased, the internal volume became smaller and smaller. Finally, the capsule turned into a solid gel bead when the ratio of CaCO3/alginate reached 4%. The dried capsule with a known weight was immersed into water to reach its equilibrium state and the weight of the saturated capsule was measured. The absorption rate of capsule was calculated using eqn (1). The relationship between water absorption and internal structure is indicated in Fig. 5D. The weight ratios for the capsules shown in Fig. 5A to C were 581%, 453% and 243%, respectively. This suggests that for the capsules shown in Fig. 5A to C were capable of absorbing 481%, 353% and 143% times their weight in water.
Fig. 6 shows the release rates of BSA from the capsules with different internal volumes. For all the three capsules, the release rates decreased with increasing release time and reached the maximum after 15 hours of incubation. Moreover, Fig. 6 also shows the influence of internal volume to drug release. When the release rates were normalized by the value of the hollow capsule (CaCO3/alginate = 16%), it could be clearly seen that the capsules with larger internal volumes had higher drug storage than the others, and as the result, they had higher drug release rates. The drug storage of semi-hollow capsules was larger than that of the solid ones. As internal volumes decreased, the amount of drug release reduced quickly. Drug release rates of semi-hollow and solid capsules were about 76% and 47% times that of hollow capsules.
 | ||
| Fig. 6 BSA release kinetics as a function of time for three different capsules: (■) hollow capsule shown in Fig. 5, (●) semi-hollow capsule, (▲) solid capsule. The weight ratio of CaCO3/alginate during the preparation of the capsules shown in A, B and C were 16%, 8% and 4% respectively. | ||
Conclusions
In summary, alginate/PAAm capsules with stretchable and tough properties were synthesized. CaCO3 microspheres played the role of both the sacrificial template for the covalent crosslinking of the PAAm network and the source of Ca2+ for the ionic crosslinking of the alginate network. The capsule had a ball-loading-ball structure and the cavity of the capsule depended on the number of the CaCO3 microspheres used during the preparation. The most important finding is that the volume of capsules could be expanded at least 27 times their original size without breakage. As a consequence, the capsules have remarkable drug loading capabilities and their drug release behavior depended on the internal volume, which was inversely proportional to the number of CaCO3 microspheres used during the preparation. This composite capsule with improved mechanical strength may have distinctive applications for drug delivery in biomedical processes.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) with grant number 21174029 and the Industry Academia Cooperation Innovation Fund of Jiangsu Province with grant number BY2014127-07 to Y.J.References
- Y. Lu, Y. Mei, M. Ballauff and M. Drechsler, J. Phys. Chem. B, 2006, 110, 3930–3937 CrossRef CAS PubMed.
- Y. Lu, A. Wittemann and M. Ballauff, Macromol. Rapid Commun., 2009, 30, 806–815 CrossRef CAS PubMed.
- A. Pich, J. Hain, Y. Lu, V. Boyko, Y. Prots and H. J. Adler, Macromolecules, 2005, 38, 6610–6619 CrossRef CAS.
- K. Iwai, Y. Matsumura, S. Uchiyama and A. P. de Silva, J. Mater. Chem., 2005, 15, 2796–2800 RSC.
- K. Jono, H. Ichikawa, M. Miyamoto and Y. Fukumori, Powder Technol., 2000, 113, 269–277 CrossRef CAS.
- H. Ichiura, M. Morikawa and K. Fujiwara, J. Mater. Sci., 2005, 40, 1987–1991 CrossRef CAS PubMed.
- K. Landfester, Adv. Mater., 2001, 13, 765–768 CrossRef CAS.
- G. T. Vladisavljevic and R. A. Williams, Adv. Colloid Interface Sci., 2005, 113, 1–20 CrossRef CAS PubMed.
- A. Jaworek, J. Microencapsulation, 2008, 25, 443–468 CrossRef CAS.
- B. Thu, P. Bruheim, T. Espevik, O. Smidsrod, P. SoonShiong and G. SkjakBraek, Biomaterials, 1996, 17, 1031–1040 CrossRef CAS.
- B. Thu, P. Bruheim, T. Espevik, O. Smidsrod, P. SoonShiong and G. SkjakBraek, Biomaterials, 1996, 17, 1069–1079 CrossRef CAS.
- S. H. Chen, S. R. Wang, S. G. Bian and X. G. Li, in Multi-Functional Materials and Structures II, Pts 1 and 2, ed. Y. S. Yin and X. Wang, Trans Tech Publications Ltd, Stafa-Zurich, 2009, vol. 79–82, pp. 381–384 Search PubMed.
- X. M. Tong, M. Zhang, M. S. Wang and Y. Fu, J. Appl. Polym. Sci., 2013, 127, 3954–3961 CrossRef CAS.
- S. M. Chia, A. C. A. Wan, C. H. Quek, H. Q. Mao, X. Xu, L. Shen, M. L. Ng, K. W. Leong and H. Yu, Biomaterials, 2002, 23, 849–856 CrossRef CAS.
- Y. P. Ma and B. R. Zhu, Cem. Concr. Res., 2009, 39, 90–94 CrossRef CAS PubMed.
- T. Sun, M. L. H. Chan, C. H. Quek and H. Yu, J. Biotechnol., 2004, 111, 169–177 CrossRef CAS PubMed.
- R. Tian, X. L. Fu, Y. D. Zheng, X. Liang, Q. L. Wang, Y. Ling and B. S. Hou, J. Mater. Chem., 2012, 22, 25437–25446 RSC.
- J. P. Gong, Y. Katsuyama, T. Kurokawa and Y. Osada, Adv. Mater., 2003, 15, 1155–1158 CrossRef CAS.
- K. J. Henderson, T. C. Zhou, K. J. Otim and K. R. Shull, Macromolecules, 2010, 43, 6193–6201 CrossRef CAS.
- R. E. Webber, C. Creton, H. R. Brown and J. P. Gong, Macromolecules, 2007, 40, 2919–2927 CrossRef CAS.
- J. Y. Sun, X. H. Zhao, W. R. K. Illeperuma, O. Chaudhuri, K. H. Oh, D. J. Mooney, J. J. Vlassak and Z. G. Suo, Nature, 2012, 489, 133–136 CrossRef CAS PubMed.
- D. V. Volodkin, A. I. Petrov, M. Prevot and G. B. Sukhorukov, Langmuir, 2004, 20, 3398–3406 CrossRef CAS.
- Y. Z. Tian, Y. L. Li, Z. F. Wang and Y. Jiang, J. Mater. Chem. B, 2014, 2, 1667–1672 RSC.
- A. Fujii, T. Maruyama, Y. Ohmukai, E. Kamio, T. Sotani and H. Matsuyama, Colloids Surf., A, 2010, 356, 126–133 CrossRef CAS PubMed.
- K. F. Ren, Y. X. Wang, J. Ji, Q. K. Lin and J. C. Shen, Colloids Surf., B, 2005, 46, 63–69 CrossRef CAS PubMed.
- N. Yamazaki, K. Naganuma, M. Nagai, G. H. Ma and S. Omi, J. Dispersion Sci. Technol., 2003, 24, 249–257 CrossRef CAS.
- C. Kim, S. Chung, Y. E. Kim, K. S. Lee, S. H. Lee, K. W. Oh and J. Y. Kang, Lab Chip, 2011, 11, 246–252 RSC.
- Q. X. Gao, C. Y. Wang, H. X. Liu, C. H. Wang, X. X. Liu and Z. Tong, Polymer, 2009, 50, 2587–2594 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 shows the SEM images of CaCO3 microspheres. Fig. S2 presents the FTIR spectra of crosslinked capsules, alginate and PAAm. Fig. S3 demonstrates the recovery process of a stretched alginate/PAAm capsule. See DOI: 10.1039/c5ra03465e |
| This journal is © The Royal Society of Chemistry 2015 |





