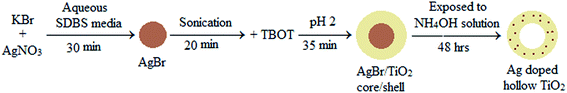Visible light induced enhanced photocatalytic degradation of organic pollutants in aqueous media using Ag doped hollow TiO2 nanospheres†
Siddhartha Sankar Boxi and
Santanu Paria*
Interfaces and Nanomaterials Laboratory, Department of Chemical Engineering, National Institute of Technology, Rourkela 769 008, Orissa, India. E-mail: santanuparia@yahoo.com; sparia@nitrkl.ac.in; Fax: +91 661 246 2999
First published on 20th April 2015
Abstract
The silver doped hollow TiO2 (Ag-h-TiO2) nanoparticles were synthesized by a sacrificial core (AgBr) method. The Ag doping and the core removal was done simultaneously during the dissolution of the core in (NH4)OH solution. The mean particle size of synthesized Ag-h-TiO2 nanoparticles is 17.76 ± 2.85 nm with a wall thickness of ∼2.5 nm. The hollow structured nanoparticles have 103.2 m2 g−1 more specific surface area compared to solid TiO2 nanoparticles. The suitability of these synthesized hollow nanoparticles as photocatalyst were tested for the photocatalytic degradation of three important different classes of organic compounds such as nitrobenzene (NB), metronidazole (MTZ) antibiotic, and methylene blue dye (MBD) in aqueous solution under irradiation of visible light. Photodegradation studies show there is a significant enhancement of the degradation efficiency of the TiO2 after the hollow structure formation and silver doping. The recycling tests of the catalysts show only ∼10% decrease in efficiency for NB and MTZ degradation after sixth cycle of reuse. The light emission capacity in terms of quantum yield (QY) is enhanced by 18.7% for Ag-h-TiO2 than that of pure TiO2 nanoparticles.
1 Introduction
Metal oxide semiconductor nanoparticles play an important role in different areas of science and engineering such as photo catalysis,1–3 solar cells,4–6 Li-ion batteries,7,8 piezoelectric devices,9,10 fuel cells,11 sensors,12,13 and so on. The photo catalytic degradation of organic pollutants is considered as an advanced environmental remediation process in recent years over conventional processes such as membrane filtration, adsorption. Among several metal oxide semiconductor nanoparticles TiO2 is a promising photocatalyst because of its relatively higher efficiency, low toxicity, long term stability, low cost, photoinduced strong oxidation activity properties.14–16 However, the application of pure TiO2 as a photocatalyst is limited in visible light because of its high band gap (3.03 eV for rutile and 3.18 eV for anatase). Because of this reason, the development of visible light induced TiO2 photocatalyst with higher efficiency is highly essential to make the industrial process more feasible and economic. Since the reduction of band gap enhance the photocatalytic activity of a photocatalyst under visible light, significant efforts have been made till now to dope different elements into the TiO2 host lattices such as, Fe,17,18 Mn,19 Mg,20 Cr,21 Co,22 Sn,23 Sm,24 Nd,25 and so on. In addition to the band gap of a photocatalyst, the surface area is also extremely important to enhance the activity of a catalyst. In this regard, TiO2 with different morphologies such as nanowires,26 nanotubes,27 nanorods28 have been studied. More advanced material like hollow nanoparticles along with doping have attracted considerable interests because of its larger surface area along with some important properties, such as low density, good surface permeability and high light-harvesting efficiencies,29–32 The TiO2 photocatalysts are not only used for the remediation of contaminated water but also useful in air purification,33 cathodic catalyst for low-cost fuel cell.34 Some studies are also available on hollow TiO2 nanoparticles doped with different elements such as Nd,35 N and Ce,36 Sn,37 Bi,38 in all these studies the C was used as the core and it was removed by calcination. The other template such as polystyrene was used for Sm3+ doped hollow TiO2 structure.39 Sulfur doped hollow sphere TiO2 was also synthesized by our group, where S was doped during the removal of the sacrificial S core.40 Considering catalytic properties of TiO2, incorporation of noble metal like Ag to hollow TiO2 structure is important because of its unique characteristics in resonant collective oscillations of the conduction electrons by electromagnetic radiation and the localized surface plasmon resonance (SPR).41,42 Since nanoscale silver has good antibacterial property,43,44 Ag doped TiO2 nanoparticles also show similar property.45–47 Silver–TiO2 composite hollow nanoparticles was reported by template free method48 and PSA latex templated method49 as a visible light active photocatalyst. Recently, Ag and AgCl doped TiO2 hollow nanoparticles were synthesized using polystyrene particles as template for getting a visible light active photocatalyst.50It has been noted from most of the literature that the doped hollow structure was formed after removing the core, and the doping was achieved in the presence of some extra precursor. However, a single step method for the core removal and doping could make the process simple.
As mentioned before, a significant fraction of research activities is going on environmental remediation of organic pollutants using TiO2 nanoparticles. The main advantage of this process over the other conventional separation processes is toxic pollutants can be converted to useful or nontoxic compounds. Different classes of organic compounds such as aromatics (benzo compounds, phenolic compounds, naphthalene, trinitrotoluene, etc.), pharmaceutical products (antibiotics, antipyretic etc.), dyes (synthetic dyes, reactive dyes, azo dyes, basic cationic dyes etc.), some volatile organic compounds (formaldehyde, methylene chloride, ethylene glycol, etc.) can be degraded using this catalysts. The benzo compounds such as nitrobenzene are highly toxic organic compound mainly used for the production of aniline, paper and pulp, pesticides, dyes, explosives, cosmetics, pharmaceuticals, and so on.51–53 The long term exposure of nitrobenzene to the environment, even at low concentration, causes risks to human, such as liver or kidney damage, lung irritation, increase heart rate, skin problem, vomiting, etc. Therefore, removal of nitrobenzene from the environment is a major concern. Degradation of nitrobenzene in effluent water is difficult by conventional chemical method because of the nitro group which has strong electron withdrawing property and inhibits its oxidation, or by biological method because of its toxic and mutagenic effect on the biological systems.54,55 Apart from aromatic compounds, the presence of pharmaceutical products for instance antibiotics in the environment, even at low concentrations, causes the growth of antibiotic-resistant bacteria56,57 and creates microbial population,58,59 which may cause of ineffectiveness of the present forms of treatment and major epidemics. Metronidazole (MTZ) is one such type of antibiotic which is primarily used for the infectious diseases caused by anaerobic bacteria and protozoa. Complete removal of metronidazole from the environment by conventional method is difficult because of its low degradability and high solubility in water. Dye contamination is another critical environmental problem and addressed by several researchers till now. The sources of synthetic dyes in wastewater are from different industries such as, textile, dye and dye intermediates, paper and pulp, printing, colour photography, petroleum industries, and so on.60,61 Continuous discharge of dye-bearing effluents from these industries into natural stream and rivers poses severe environmental problems as toxic to useful microorganisms, aquatic life, and human beings. So, suitable and efficient techniques are highly essential for the treatment of these industrial effluents.
In this study Ag-h-TiO2 nanoparticles were synthesized through a sacrificial core technique using AgBr as the core. The core was removed under a mild condition by dissolving in ammonium hydroxide solution, and the silver doping was also achieved during the dissolution of the core without any addition extra dopant precursor. After Ag doping, pure anatase phase TiO2 particles were obtained even after heating at 450 °C. The hollow structure nanoparticles have higher surface area (198.3 m2 g−1) compared to solid TiO2 nanoparticles (95.1 m2 g−1) and hollow structures reported by other researchers.62–66 To test the photocatalytic activity of the developed nanoparticles towards three important organic compounds such as nitrobenzene (NB), metronidazole (MTZ), and methylene blue dye (MBD) degradation studies were conducted with low catalyst dose and high initial MTZ concentration compared to the studies reported till now. The obtained TiO2 particles are having low band gap, anatase phase, high surface area, good crystallinity, and efficient visible light induced photocatalytic property to degrade important organic compounds such as nitrobenzene, antibiotic, and dye.
2 Materials and methods
2.1 Materials
Reagent-grade silver nitrate (99.9%) and potassium bromide (99.3%) were purchased from Rankem. Anionic surfactant sodium dodecyl benzene sulphonate (SDBS) (technical grade, Cat no. 28995-7) and titanium(IV) butoxide (Ti[O(CH2)3CH3]4 or TOBT, 97%) were obtained from Sigma Aldrich. Ammonium hydroxide (25%), nitric acid (69% GR), cyclohexane, and methylene blue dye were bought from Merck. Nitrobenzene was provided by Nice Chemicals Pvt. Ltd. Metronidazole was supplied by J B Chemicals & Pharmaceuticals Ltd. All the chemicals were used as it was received without any further purification. Ultrapure water of 18.2 MΩ cm resistivity and pH 6.4–6.5 was used for all the experiments.2.2 Methods
A sol–gel method was followed for the synthesis of silver doped hollow TiO2 nanoparticles using AgBr as the sacrificial core (Scheme 1). The AgBr nanoparticles were synthesized in aqueous SDBS media according to our previous reported study.67 The concentrations of both AgNO3 and KBr were maintained at 0.1 mM. After the formation of AgBr NPs the suspension was sonicated using a bath sonicator for 20 min, TBOT was then added slowly under constant stirring condition for uniform coating on the core surface. After 5 minutes of addition of TBOT, HNO3 was added to maintain the pH of ∼2 and kept for 35 min under stirring condition. The AgBr/TiO2 core/shell nanoparticles were then separated by centrifugation at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min and washed thrice with ethanol and water mixture (2
000 rpm for 20 min and washed thrice with ethanol and water mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Then the particles were dipped in ammonium hydroxide solution (2.5 mM) for 48 h for the dissolution of AgBr core. The particles were separated and calcined at 450 °C for 2 h to improve the crystallinity. For quantum yield (QY) calculation of Ag-h-TiO2 NPs, phenol was used as a reference (QY = 0.14).
1, v/v). Then the particles were dipped in ammonium hydroxide solution (2.5 mM) for 48 h for the dissolution of AgBr core. The particles were separated and calcined at 450 °C for 2 h to improve the crystallinity. For quantum yield (QY) calculation of Ag-h-TiO2 NPs, phenol was used as a reference (QY = 0.14).
2.3 Particles characterization
The particle size was measured initially by dynamic light scattering (DLS) using a Malvern Zeta Size analyzer, (Nano ZS). The crystallinity of synthesized particles was characterized using powder X-ray diffraction (XRD) (Philips, PW 1830 HT) with scanning rate of 0.01° s−1 in the 2θ range from 20° to 70°. The size and shape of the particles were observed under a field emission scanning electron microscope (FE-SEM) (FEI, Nova NanoSEM NPE212) and transmission electron microscope (TEM) (FEI, Tecnai S-twin). The elemental composition of the sample was analyzed by energy-dispersive X-ray spectroscopy (EDX) (Oxford Instruments, model X-sight) attached to the HR-TEM (JEOL, JEM 2100). The light absorbance and luminescence properties were also characterized by UV-vis-NIR Spectroscopy (Shimadzu, UV-3600) and fluorescence spectroscopy (Hitachi, F-7000) respectively. Fourier transform infrared spectroscopy (FT-IR) was carried out using an FT-IR (Thermo Fisher Scientific, Nicolet iS10). The chemical composition of the samples and the valence states of various elements were analyzed by X-ray photoelectron spectroscopy (XPS, ULVAC-PHI, Inc., PHI 5000 Versa Probe II.). The surface area of the nanoparticles was measured by Brunauer–Emmett–Teller (BET) technique (Quantachrome, USA).2.4 Photocatalytic degradation
The photocatalytic activity of the synthesized catalyst was tested by the degradation of NB, MTZ antibiotic, and MBD in aqueous solutions. For nitrobenzene degradation the initial concentration of nitrobenzene and catalyst dose were maintained 61.5 mg L−1 and 0.05 g L−1. For MTZ degradation the initial concentration of the MTZ solution was maintained to 15 mg L−1 for all experiments and the catalyst concentration was 0.5 g L−1. The degradation studies were conducted in a photoreactor equipped with a 125 W high pressure mercury vapour lamp (λ = 435.8 nm, light intensity 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 LUX, light energy 56 ± 2 W m−2) placed about 12 cm away from the solution. The solutions were stirred continuously using a magnetic stirrer. Prior to each test, the lamp was turned on 10 min before in order to get a maximum light intensity. The samples were magnetically stirred for 20 min in the dark to allow physical adsorption equilibrium of NB, MTZ, and MBD on the catalyst surface. Then the suspension was exposed to visible light irradiation under constant stirring condition. The sample was then taken out and centrifuged to remove the nanoparticles after each 20 min interval for MTZ and 30 min interval for NB and MBD degradation. Then the concentration of the NB, MTZ, and MBD solution were analyzed using a UV-Vis-NIR Spectrophotometer at its maximum absorbance wavelength of λ = 267, 320, and 661 nm for NB, MTZ, and MBD, respectively. The cyclic degradation test was conducted to check the reusability of the photocatalyst. After each cycle of degradation the catalyst was separated by centrifugation and was used for the next cycle of degradation without any pre-treatment. The intermediate and final degraded products during nitrobenzene degradation were analyzed by liquid chromatography-mass (LC-MS) (Flexar SQ 300 MS, Perkin Elmer). The sample was extracted with cyclohexane and 70% water/30% acetonitrile (v/v) mobile phase was used for the analysis.
000 LUX, light energy 56 ± 2 W m−2) placed about 12 cm away from the solution. The solutions were stirred continuously using a magnetic stirrer. Prior to each test, the lamp was turned on 10 min before in order to get a maximum light intensity. The samples were magnetically stirred for 20 min in the dark to allow physical adsorption equilibrium of NB, MTZ, and MBD on the catalyst surface. Then the suspension was exposed to visible light irradiation under constant stirring condition. The sample was then taken out and centrifuged to remove the nanoparticles after each 20 min interval for MTZ and 30 min interval for NB and MBD degradation. Then the concentration of the NB, MTZ, and MBD solution were analyzed using a UV-Vis-NIR Spectrophotometer at its maximum absorbance wavelength of λ = 267, 320, and 661 nm for NB, MTZ, and MBD, respectively. The cyclic degradation test was conducted to check the reusability of the photocatalyst. After each cycle of degradation the catalyst was separated by centrifugation and was used for the next cycle of degradation without any pre-treatment. The intermediate and final degraded products during nitrobenzene degradation were analyzed by liquid chromatography-mass (LC-MS) (Flexar SQ 300 MS, Perkin Elmer). The sample was extracted with cyclohexane and 70% water/30% acetonitrile (v/v) mobile phase was used for the analysis.
3 Results and discussion
3.1 Synthesis of hollow TiO2 particles
The hollow particles were synthesized by the templated sacrificial core method using AgBr as a core. In aqueous media the surface charge of AgBr nanoparticles is −15 to −25 mV. Addition of anionic surfactant (SDBS) makes the surface charge of AgBr more negative (−47.8 mV). The surface charge of TiO2 nanoparticles is positive, so, it is expected that the TiO2 will favourably coat on the AgBr core surface because of the electrostatic attraction. When ammonium hydroxide solution was added to the particle suspension, AgBr would try to dissolve in ammonium hydroxide through the formation of soluble complex according to eqn (1). During the dissolution of AgBr, there is a chance of doping of Ag on TiO2 shell.| AgBr (s) + 2NH3 (aq) → Ag(NH3)2+ (aq) + Br− | (1) |
3.2 Characterization of particles by UV-Vis spectroscopy
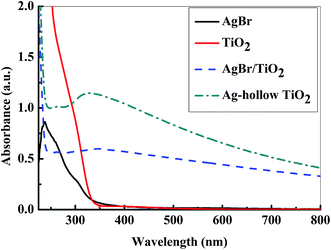
UV-Vis spectroscopic study was done to investigate the light absorption property of the nanoparticles, and the results are presented in Fig. 1. The figure shows that the sharp peak of pure AgBr is present in the UV region (237 nm), and it disappears after the coating of external shell layer of TiO2 on AgBr. Pure TiO2 is having major absorption below 350 nm wavelength, and there is no absorption in the visible region, this is because of its high energy band gap (3.2 eV). However, the UV-Vis spectra of Ag-h-TiO2 nanoparticles show a significant absorption in 300–650 nm wavelength range. The shifting of absorbance in the visible range is attributed to the fact of lowering of the band gap of the obtained nanoparticles because of doping of Ag. In addition, noteworthy to mention that, the absorption intensity of Ag-h-TiO2 is higher compared to that of pure solid TiO2 in both UV as well as in visible regions. The increase in absorbance is also attributed to the fact of porous hollow structure and formation of shell layer by the deposition of small particles. The band gap energy was calculated using the equation Eg = 1239.8/λ (ref. 68) to support the above fact, where Eg is the band gap (eV) and λ is the wave length (nm) of the absorption edges in the UV-Vis spectrum. It has been found that the calculated band gaps are 2.69 and 3.29 eV for AgBr and TiO2, which are in good agreement with the reported values of 2.7 eV (ref. 69) and 3.28 eV (ref. 70) respectively, whereas, 2.48 and 2.25 eV for AgBr/TiO2 and Ag-h-TiO2 nanoparticles respectively. Since the band gap of the TiO2 is reduced after the formation of doped hollow structure, it is expected to be useful as good photocatalyst under the visible light irradiation, dye sensitized solar cells, or other electrochemical applications.3.3 Characterization of particles by XRD
The crystallographic phases of the nanoparticles were identified by XRD technique. The diffraction patterns of all particles are presented in Fig. 2. For pure AgBr, the XRD pattern displays peaks at 2θ value of 26.6, 30.9, 44.3, 55.2, 64.5, and 73.3° corresponding to the planes (111), (200), (220), (222), (400), and (420) of cubic structure (JCPDS card no. 79-0149). For pure TiO2, the anatase peaks were obtained at 2θ value of 25.1, 36.5 and 62.3° corresponding to the planes (101), (103), and (204) (JCPDS card no. 71-1169). The peaks rutile phase were obtained at 2θ values of 27.3, 54.1, and 68.6° corresponding to the planes (110), (211), and (301) (JCPDS card no. 76-0319). For AgBr/TiO2 core/shell nanoparticles the XRD pattern shows the peaks of both AgBr and TiO2, but the peak intensity of AgBr is reduced and that of TiO2 becomes more dominant mainly because of TiO2 coating. For Ag-h-TiO2 NPs the TiO2 anatase peaks (2θ) were obtained at 25.6, 37.4, 48.6, and 63.2° corresponding to the planes of (101), (103), (200), and (204) (JCPDS card no. 75-1537) with the peaks of elemental silver at 2θ values of 44.3 and 64.7, corresponding to the planes (200) and (220) (JCPDS card no. 03-0931) respectively. Additionally, another important observation is the rutile peak at 2θ = 27.3° is absent in the hollow structure, while compare with pure TiO2 NPs. The XRD analysis also shows that the anatase TiO2 peak (2θ) of (101) plane shifts from 25.1° to 25.6° after the formation of hollow structure, which attributed to the silver doping to TiO2 lattices.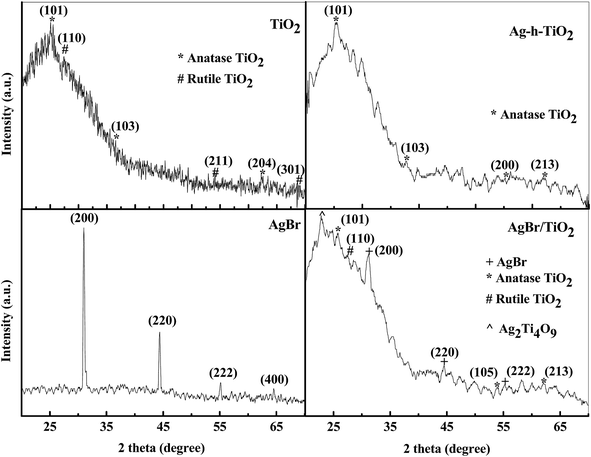 | ||
| Fig. 2 The X-ray diffraction patterns of AgBr, TiO2, AgBr/TiO2 core/shell, and Ag-h-TiO2 nanoparticles. | ||
3.4 Characterization of particles by TEM and FE-SEM
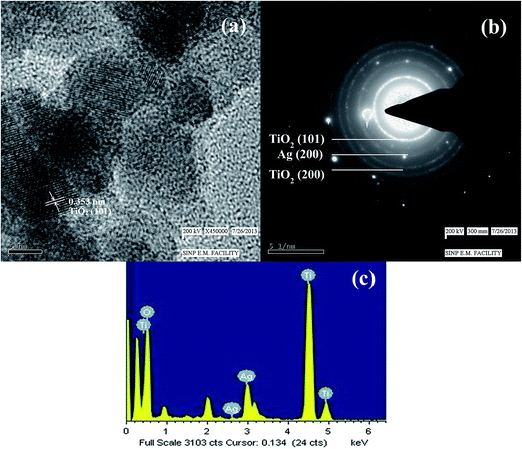
The Fig. 3a shows the TEM image of AgBr/TiO2 core/shell nanoparticles before expose to ammonium hydroxide solution. The contrast difference between the core and shell in the image indicates the formation of core/shell structure. The particles are spherical in shape, and the size distribution is plotted in Fig. 3c which indicates the mean particle size of 17.86 ± 2.46 nm and the shell thickness is ∼2.5 nm. The TEM image of AgBr/TiO2 core/shell particles after dissolution in ammonium hydroxide solution is presented in Fig. 3b. The particle size distribution is plotted in Fig. 3d which shows mean particle size of 17.76 ± 2.85 nm, which is almost same as it was before the dissolution. However, the average particle size obtained by DLS measurement (S1, ESI†) was comparatively larger (20.79 nm) than that obtained by TEM measurement, this might be because of the adsorbed surfactant layer and hydrated water molecules.67 The FE-SEM image (inset Fig. 3b and S2, ESI†) clearly shows some of the broken hollow spheres. The TEM image of single contrast indicates the removal of core. So, the TEM and FE-SEM images confirm the formation of hollow TiO2 particles after treatment with ammonia solution. The high resolution TEM image of Ag-h-TiO2 nanoparticles (Fig. 4a) shows lattice fringes spacing of 0.353 nm corresponding to the (101) plane for anatase phase TiO2 (JCPDS card no. 75-1537) previously confirmed by XRD. But there is no lattice fringe of rutile TiO2 or Ag, which confirms 100% anatase phase of TiO2 and no surface deposition of Ag. The Fig. 4b shows the selected area electron diffraction (SAED) pattern of the hollow particle by focusing the electron beam on a single particle. The ring like diffraction pattern is indexed to (101) and (200) planes of TiO2 together with (200) diffraction of silver, which confirms the presence of silver inside the crystal structure of TiO2 nanoparticles. The EDX in Fig. 4c further confirms the presence of silver, titanium, and oxygen atoms. Some extra peaks of Cu and C are mainly from TEM grids.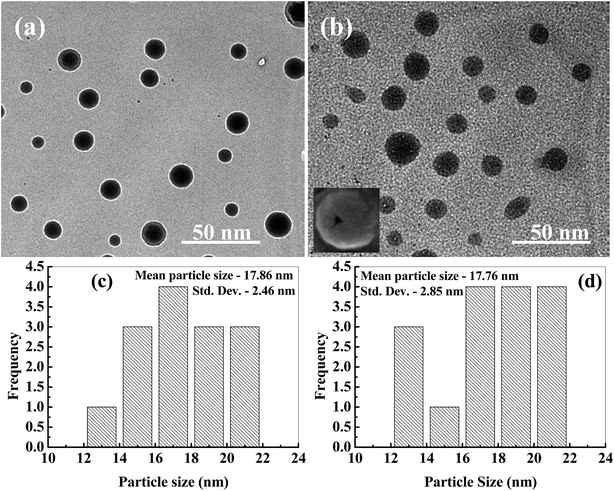 | ||
| Fig. 3 TEM images of (a) AgBr/TiO2 core/shell, and (b) Ag-h-TiO2 hollow nanoparticles, inset shows FE-SEM image of a hollow broken particle (more hollow particles are shown in Fig. S2†). Particle size distributions of (c) AgBr/TiO2 core/shell, and (d) Ag-h-TiO2 nanoparticles. | ||
3.5 Characterization of particles by XPS
For the further confirmation of the surface composition and the chemical states of Ag-h-TiO2 nanoparticles, XPS analysis was carried out. The high resolution XPS spectra of Ag (3d), Ti (2p) and O (1s) are presented in Fig. 5. The XPS peak at 462.9 eV corresponds to Ti 2p1/2, and 457.2 eV corresponds to Ti 2p3/2 in the Ti 2p region (Fig. 5a). The slitting between Ti 2p1/2 and Ti 2p3/2 is 5.9 eV which indicates the normal state of Ti4+ in the mesoporous anatase TiO2.71 The XPS peak (Fig. 5b) at ∼530 eV corresponds to the lattice oxygen of TiO2 crystal and peaks at 532.1 and 531.7 eV correspond to the adsorbed oxygen on TiO2 surface. Fig. 5c shows the Ag 3d3/2 and Ag 3d5/2 peaks at the binding energy of 366.5 and 372.6 eV, respectively with a slitting of the 3d doublet of 6.1 eV, which suggest the zero valence silver instead of Ag+ ions within the material.72 So, the possible location of dopant is attributed as the surface or in the interstitial sites of the host material. The TEM or FE-SEM images confirm that there is no surface deposition of the silver on the particles. So, there is a fair possibility of the silver doping in the interstitial sites of the host material.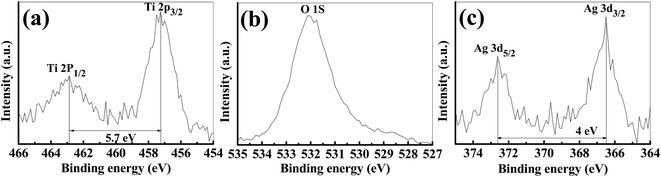 | ||
| Fig. 5 High resolution XPS narrow scan spectra of (a) Ti(2p), (b) O (1s), and (c) Ag (3d) for Ag-h-TiO2 nanoparticles. | ||
3.6 Characterization of particles by BET analysis
The specific surface area of the pure and hollow doped TiO2 nanoparticles was measured by BET technique. Before the adsorption–desorption study the sample was degasified for 1 h at 150 °C temperature. The BET surface area of solid TiO2 and Ag-h-TiO2 nanoparticles are 95.1 and 198.3 m2 g−1 respectively. The Ag-h-TiO2 nanoparticles are having 103.2 m2 g−1 more surface area compare to that of solid TiO2. The obtained surface area of hollow structure TiO2 is higher compared to the literature values of 38.9–123 m2 g−1 (ref. 62–66) This might be because of the core removal technique and the final particle size. In most of the literature the core was removed by calcinations but in our case the core is removed by dissolution at room temperature. The high surface area hollow structure TiO2 is expected to be useful for the catalysis or other applications.3.7 Photoluminescence activity of the synthesized nanoparticles
The photoluminescence (PL) properties of the synthesized nanoparticles were also checked by the fluorescence spectroscopy in terms of light emission. The light emission data was recorded between 500–600 nm wavelengths while excitation at 270 nm. The emission spectra of AgBr, TiO2, AgBr/TiO2, and Ag-h-TiO2 NPs are presented in Fig. 6. All the particles show emission in the visible region of wavelength ∼543.8 nm. The intensities of emission are in the following order: AgBr > Ag-h-TiO2 > AgBr/TiO2 > TiO2. The pure TiO2 have very low intensity compared to pure AgBr and the intensity increases after the formation of Ag-h-TiO2 NPs. The quantum yield (QY) was calculated for these materials from UV and PL data (eqn (SI1), ESI†) using phenol as the standard material (QY = 0.14). The QYs of TiO2, AgBr, AgBr/TiO2, and Ag-h-TiO2 NPs are 0.29, 0.498, 0.36, and 0.477 respectively. So, the quantum yield of TiO2 is enhanced by 18.7% after the formation of hollow structure and Ag doping. From these results it can be pointed out that the Ag-h-TiO2 nanoparticles has enhanced light emission property and QY compared to that of pure TiO2 nanoparticles. So, the light emission properties along with the catalytic properties could be utilized for better photocatalyst or dye sensitized solar cells applications.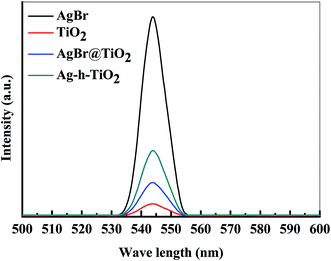 | ||
| Fig. 6 Light emission spectra of AgBr, TiO2, AgBr/TiO2, and Ag-h-TiO2 NPs after excitation at 270 nm wavelength. | ||
3.8 Photo catalytic applications of synthesized nanoparticles
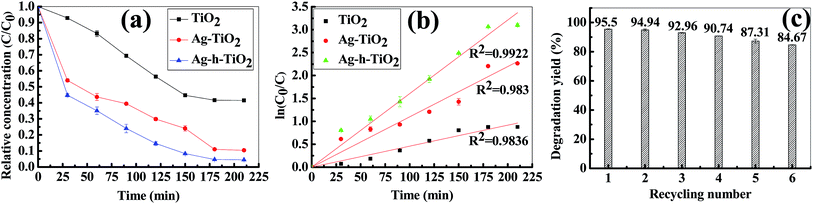
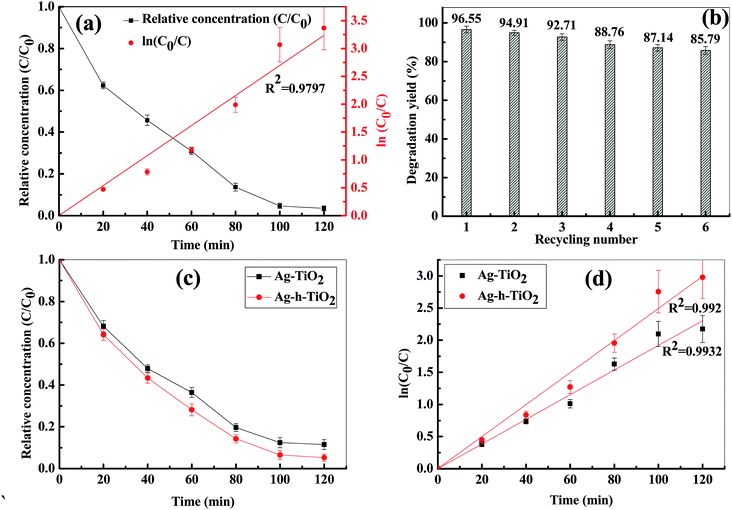
The photocatalytic activities of the synthesized Ag-h-TiO2 nanoparticles were first tested for the degradation of NB under the visible light irradiation. The degradation studies using pure and Ag doped solid TiO2 (Ag–TiO2) were also employed as the photocatalyst for the comparison purpose. The results of NB degradation in visible light are shown in Fig. 7. It has been found that the degradation process reaches a plateau level after 3.5 h of light exposure time. The results show that at the plateau level, the degradation efficiencies of NB are 58.46, 89.61, and 95.5% in the presence of TiO2, Ag–TiO2, and Ag-h-TiO2 NPs respectively. The results clearly indicate that the maximum degradation of the NB was obtained in the presence of the Ag-h-TiO2 NPs. The degradation results were also fitted with different kinetic models, and it was found that the first order kinetics fits well for all the materials. The Fig. 7b presents the reaction kinetics of NB degradation. The rate constants (k) of the photocatalytic degradation reactions obtained from the experimental results are 0.0045, 0.0109, and 0.016 min−1 for TiO2, Ag–TiO2, and Ag-h-TiO2 NPs respectively. The rate constant of Ag-h-TiO2 photocatalytic process is about 3.6 and 1.5 times than that of pure TiO2 and Ag–TiO2 photocatalytic processes respectively. A comparison at 50% degradation times for NB shows 136.5, 41.5, and 27 min for TiO2, Ag–TiO2, and Ag-h-TiO2 nanoparticles, respectively. These results reveal that Ag-h-TiO2 nanoparticles have higher photocatalytic activity compared to that of pure TiO2 and Ag–TiO2 NPs. This is attributed to the fact that pure TiO2 is having low yield of ˙OH radicals because of recombination of the charge carriers during photodegradation, which helps to oxidize the organic compound as stated in our previous study.73 In Ag doped TiO2 NPs the Ag dopant accepts the photoinduced electrons and holes, which in turn act as electron/hole traps and prevents the recombination of the charge carriers. In Ag-h-TiO2 NPs, addition to Ag doping it is having more surface area (198.3 m2 g−1 as mentioned in the BET analysis) compared to solid TiO2 (95.1 m2 g−1), which is useful for better catalytic processes. Additionally, as mention in the XPS section, the presence of adsorbed oxygen on the TiO2 surface allows the H+ hydroxylation to form –Ti(OH)–O–Ti–(OH)– in the presence of water. During the photodegradation, this compound may help to generate the photogenerated hole (h+VB) and finally turn to ˙OH free radicals, which is beneficial for the photocatalysis.74 While comparing the degradation efficiency and PL intensity, it can be seen that Ag-h-TiO2 is having high degradation efficiency as well as PL intensity compared to that of pure TiO2. In general, high PL intensity is due to the high recombination of electrons and holes. Our results can be attributed in the following way. In the presence of doping, the reduction in recombination continues up to the certain limit of doping percentage (optimum doping) and then again recombination starts increasing with the increasing doping %.75,76 From the EDX study it is seen that 9.02% doping is achieved, which is higher compared to optimum doping percentage (1%) for TiO2,73 we believe because of that the PL intensity is high. At the same time, as the surface area of hollow particles is higher than solid TiO2, the degradation efficiency is more because of more adsorption of dye molecules. The reusability of Ag-h-TiO2 NPs was tested by recycling the catalyst under the same condition, and the results are plotted in Fig. 7c. The results show there is a ∼10.83% decrease (95.5 to 84.67%) in degradation efficiency after sixth cycles. The turn over number (TON) of the photocatalyst is calculated as 0.467, 0.716, and 0.763 mol mol−1 for the first cycle of the TiO2, Ag–TiO2, Ag-h-TiO2, respectively. From the reported literatures (Table S1, ESI†) it is has been found that more than 90% degradation efficiency of nitrobenzene was achieved either in the presence high catalyst dose, low organic concentration, in the presence of UV light irradiation, or in presence of some additives and at specific pH. In this regard, our catalytic process is more effective in normal visible light and in the absence of any additives than the already reported literatures.Further, to explore the degradation pathway of NB in the presence of TiO2, Ag–TiO2, and Ag-h-TiO2 photocatalysts, LC-MS study was conducted to identify different photo degraded products. Based on the LC-MS results probable degradation path is proposed in Fig. 8. During the photodegradation, the ˙OH radicals attack the nitrobenzene and subsequently produce some aromatic compounds, such as benzene, phenol, catechol, resorcinol, and hydroquinone. The hydroquinone again forms benzoquinone through the dehydrogenation. Further degradation of the aromatic compounds happens through the ring cleavage and some degraded product, such as propionic acid, n-butanol, di-ethyl ether, furan, orthoformic acid, propanol, and acetic acid are formed.
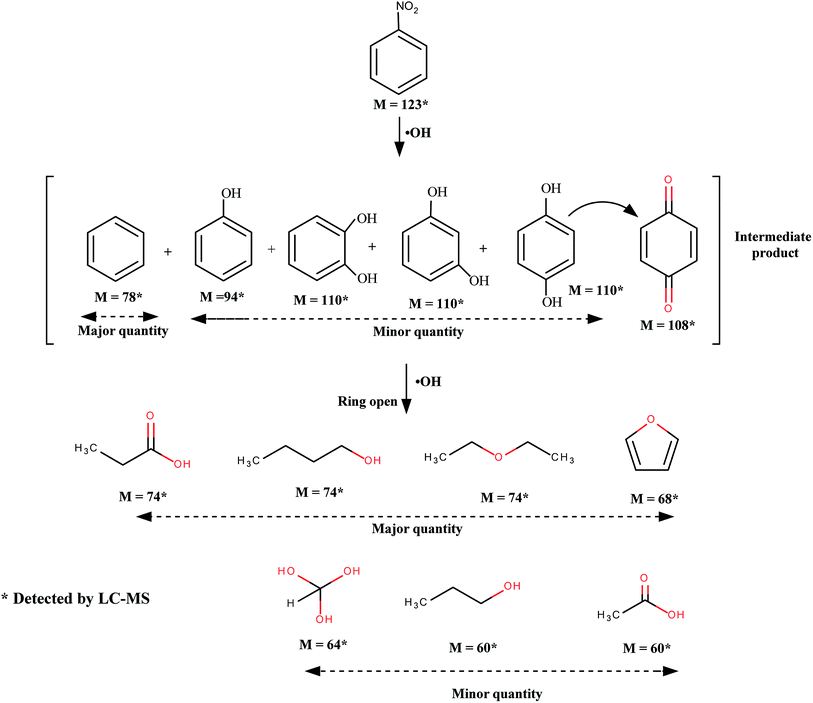 | ||
| Fig. 8 Probable pathway for the degradation of nitrobenzene under visible light in the presence of TiO2, Ag–TiO2, and Ag-h-TiO2. | ||
Since the Ag-h-TiO2 nanoparticles show good photocatalytic degradation against the NB, the same catalyst was also used to study the photodegradation efficiency on other two classes of organic compounds such as metronidazole antibiotic and methylene blue dye under the visible light irradiation. The degradation results (Fig. 9a) show that at the plateau level the degradation of MTZ is 96.55% in the presence of Ag-h-TiO2 NPs, while pure TiO2 and Ag–TiO2 degraded 80.78 and 94.39% of MTZ.73 The rate constant of the photocatalytic degradation reactions obtained from the experimental results is 0.0269 min−1 for Ag-h-TiO2 NPs. The results of reusability test of Ag-h-TiO2 NPs (Fig. 9b) show there is a ∼10.76% decrease (96.55 to 85.79%) in degradation efficiency after sixth cycles. It can be noted that the difference between hollow and solid TiO2 is not significant (∼2%), in spite of significantly high surface area for hollow particles, which can be attributed to the low initial MTZ concentration. So, the initial concentration of MTZ was increased to 30 mg L−1 and it has been found that after 2 h the Ag–TiO2 and Ag-h-TiO2 NPs degraded 88.5 and 94.77% MTZ (Fig. 9c) with rate constants 0.019 and 0.024 min−1 (Fig. 9d) for Ag–TiO2 and Ag-h-TiO2 NPs respectively. These results indicate that, when the concentration of organic molecules is high hollow particles show better efficiency. Our approach of MTZ degradation is noble compared to the reported conventional method as well as photocatalytic degradation approach as discussed in the ESI (Table S2†). The surface chemistry of Ag-h-TiO2 photocatalyst was studied before and after degradation of MTZ solution by FT-IR spectroscopy. The FT-IR results (Fig. S3, ESI†) show there is no significant peak change between the virgin Ag-h-TiO2 and recycled Ag-h-TiO2 after degradation. The broad absorption peak at 3373 and 2359 cm−1 are associated with the asymmetric and symmetric stretching vibrations of the Ti–OH group. The peaks in the range 400–1000 cm−1 are the contribution of anatase phase Ti–O. So, these results strongly suggest that the degradation of MTZ solution is due to the photocatalytic degradation instead of any chemisorption or physisorption of MTZ on the catalyst surface.
The MBD degradation was carried out under the visible light with 25 mg L−1 initial concentration of dye and the catalyst concentration of 0.25 g L−1. The degradation results are presented in Fig. S4, ESI.† The dye degradation efficiency of Ag-h-TiO2 is 96.77% after 3 h exposure time. The rate constant of photocatalytic degradation of MBD under the visible lamp using Ag-h-TiO2 NPs is 0.0216 min−1 (Fig. S4c, ESI†). Similar to previous study Ag-h-TiO2 sample was further selected for cyclic degradation test under the same condition and found there is a decrease in efficiency of ∼10.47% (96.77 to 86.3%) after sixth cycle of photodegradation (Fig. S4d, ESI†).
For each type of organic compound degradation, two sets of reference studies, (i) organic solution + catalyst in dark and (ii) only organic solution under visible light irradiation were carried out. The first was to test only adsorption capacity. Since the catalyst concentration was low, there was no significant change found because of adsorption. So we confirmed that the concentration change was only because of degradation.
4 Conclusions
The hollow TiO2 nanoparticles were synthesized by a sacrificial core method using AgBr as sacrificial core. During the removal of core, Ag was doped in the interstitial sites of the host TiO2. The obtained Ag-h-TiO2 nanoparticles are purely in anatase phase and good crystalline in nature. The mean particle size is 17.76 ± 2.85 nm with wall thickness of ∼2.5 nm. The increase of surface area for hollow nanoparticles is 103.2 m2 g−1 more compared to that of solid TiO2 nanoparticles. Apart from the surface area, the quantum yield of Ag-h-TiO2 nanoparticles also increases to 18.7% compared to that of pure solid TiO2 nanoparticles. The nanoparticles were used for the photo degradation of NB, MTZ antibiotic and MBD. The maximum NB degradation was obtained 95.5% under visible light irradiation for 3.5 h. The metronidazole degradation efficiency was found to be 96.55 and 94.77% under the irradiation of visible light for the initial MTZ concentration of 15 and 30 mg L−1 with catalyst dose of 0.5 g L−1. The Ag-h-TiO2 NPs show only 10.47% decrease in degradation efficiency even after sixth cycle of reuse. The synthesized Ag-h-TiO2 nanoparticles may also be useful for dye sensitized solar cells and other electrochemical applications.Acknowledgements
The financial support from the Council of Scientific and Industrial Research (CSIR), Grant no. 22(0527)/10/EMR-II, for this project is gratefully acknowledged. S.S.B. thanks CSIR, India, for a Senior Research Fellowship to pursue this work. Authors are grateful to Indian Institute of Technology, Kharagpur, India, for providing their XPS facility. Authors also acknowledge the Saha Institute of Nuclear Physics, Indian Association for the Cultivation of Science, Kolkata, India, for giving the opportunity to access their TEM facility.References
- J. Liqiang, F. Honggang, W. Baiqi, W. Dejun, X. Baifu, L. Shudan and S. Jiazhong, Effects of Sn Dopant on the Photoinduced Charge Property and Photocatalytic Activity of TiO2 Nanoparticles, Appl. Catal., B, 2006, 62, 282–291 CrossRef PubMed
.
- H. Zeng, W. Cai, P. Liu, X. Xu, H. Zhou, C. Klingshirn and H. Kalt, ZnO-Based Hollow Nanoparticles by Selective Etching: Elimination and Reconstruction of Metal Semiconductor Interface, Improvement of Blue Emission and Photocatalysis, ACS Nano, 2008, 2, 1661–1670 CrossRef CAS PubMed
.
- W. He, H. K. Kim, W. G. Wamer, D. Melka and J. H. Callahan, Photogenerated Charge Carriers and Reactive Oxygen Species in ZnO/Au Hybrid Nanostructures with Enhanced Photocatalytic and Antibacterial Activity, J. Am. Chem. Soc., 2014, 136, 750–757 CrossRef CAS PubMed
.
- R. Jose, V. Thavasi and S. Ramakrishna, Metal Oxides for Dye-Sensitized Solar Cells, J. Am. Ceram. Soc., 2009, 301, 289–301 CrossRef PubMed
.
- J. Guo, C. She and T. Lian, Ultrafast Electron Transfer between Conjugated Polymer and Antimony-Doped Tin Oxide (ATO) Nanoparticles, J. Phys. Chem. C, 2008, 112, 4761–4766 CAS
.
- A. Tubtimtae, K. Arthayakul, B. Teekwang and K. Hongsith, MnTe Semiconductor-Sensitized Boron-Doped TiO2 and ZnO Photoelectrodes for Solar Cell Applications, J. Colloid Interface Sci., 2013, 405, 78–84 CrossRef CAS PubMed
.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Coaxial MnO2/Carbon Nanotube Array Electrodes for High-Performance Lithium Batteries, Nano Lett., 2009, 9, 1002–1006 CrossRef CAS PubMed
.
- C. K. Chan, H. Peng, R. D. Twesten, K. Jarausch, X. F. Zhang and Y. Cui, Fast, Completely Reversible Li Insertion in Vanadium Pentoxide Nanoribbons, Nano Lett., 2007, 7, 490–495 CrossRef CAS PubMed
.
- T. Kimura, Q. Dong, S. Yin, T. Hashimoto, A. Sasaki and T. Sato, Synthesis and Piezoelectric Properties of Li-doped BaTiO3 by a Solvothermal Approach, J. Eur. Ceram. Soc., 2013, 33, 1009–1015 CrossRef CAS PubMed
.
- Q. Yang, X. Guo, W. Wang, Y. Zhang, S. Xu, D. H. Lien and Z. L. Wang, Enhancing Sensitivity of a Single ZnO Micro-/Nanowire Photodetector by Piezo-phototronic Effect, ACS Nano, 2010, 4, 6285–6291 CrossRef CAS PubMed
.
- P. V. Kamat, Graphene-Based Nanoarchitectures. Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Carbon Support, J. Phys. Chem. Lett., 2010, 1, 520–527 CrossRef CAS
.
- Y. F. Sun, S. B. Liu, F. L. Meng, J. Y. Liu, Z. Jin, L. T. Kong and J. H. Liu, Metal Oxide Nanostructures and their Gas Sensing Properties: A Review, Sensors, 2012, 12, 2610–2631 CrossRef CAS PubMed
.
- H. S. Hassan, A. B. Kashyout, H. M. A. Soliman, M. A. Uosif and N. Afify, Effect of reaction time and Sb doping ratios on the architecturing of ZnO nanomaterials for gas sensor applications, Appl. Surf. Sci., 2013, 277, 73–82 CrossRef PubMed
.
- A. Zaleska, Doped-TiO2: A Review, Recent Pat. Eng., 2008, 2, 157–164 CrossRef CAS
.
- Y. Liu, J. Li, B. Zhou, H. Chen, Z. Wang and W. Cai, A TiO2-Nanotube-Array-Based Photocatalytic Fuel Cell using Refractory Organic Compounds as Substrates for Electricity Generation, Chem. Commun., 2011, 47, 10314–10316 RSC
.
- S. Cao, K. L. Yeung and P. L. Yue, An Investigation of Trichloroethylene Photocatalytic Oxidation on Mesoporous Titania–Silica Aerogel Catalysts, Appl. Catal., B, 2007, 76, 64–72 CrossRef CAS PubMed
.
- J. Zhu, F. Chen, J. Zhang, H. Chen and M. Anpo, Fe3+–TiO2 Photocatalysts Prepared by Combining Sol–gel Method with Hydrothermal Treatment and their Characterization, J. Photochem. Photobiol., A, 2006, 180, 196–204 CrossRef CAS PubMed
.
- W. Wang, J. Zhang, F. Chen, D. He and M. Anpo, Preparation and Photocatalytic Properties of Fe3+-Doped Ag@TiO2 Core–shell Nanoparticles, J. Colloid Interface Sci., 2008, 323, 182–186 CrossRef CAS PubMed
.
- Q. R. Deng, X. H. Xia, M. L. Guo, Y. Gao and G. Shao, Mn-doped TiO2 Nanopowders with Remarkable Visible Light Photocatalytic Activity, Mater. Lett., 2011, 65, 2051–2054 CrossRef CAS PubMed
.
- M. A. Behnajady, B. Alizade and N. Modirshahla, Synthesis of Mg-Doped TiO2 Nanoparticles under Different Conditions and its Photocatalytic Activity, Photochem. Photobiol., 2011, 87, 1308–1314 CrossRef CAS PubMed
.
- K. B. Jaimy, S. Ghosh, S. Sankar and K. G. K. Warrier, An Aqueous Sol–gel Synthesis of Chromium(III) Doped Mesoporous Titanium Dioxide for Visible Light Photocatalysis, Mater. Res. Bull., 2011, 46, 914–921 CrossRef CAS PubMed
.
- M. Iwasaki, M. Hara, H. Kawada, H. Tada and S. Ito, Cobalt Ion-Doped TiO2 Photocatalyst Response to Visible Light, J. Colloid Interface Sci., 2000, 224, 202–204 CrossRef CAS PubMed
.
- N. Kiraz, E. Burunkaya, Ö. Kesmez, H. E. Çamurlu, M. Asiltürk, Z. Yeşil and E. Arpaç, Preparation of Sn Doped Nanometric TiO2 Powders by Reflux and Hydrothermal Syntheses and their Characterization, J. Sol-Gel Sci. Technol., 2011, 59, 381–386 CrossRef CAS
.
- Q. Xiao, Z. Si, Z. Yu and G. Qiu, Sol–gel Auto-combustion Synthesis of Samarium-Doped TiO2 Nanoparticles and their Photocatalytic Activity under Visible Light Irradiation, Mater. Sci. Eng., B, 2007, 137, 189–194 CrossRef CAS PubMed
.
- T. L. R. Hewer, E. C. C. Souza, T. S. Martins, E. N. S. Muccillo and R. S. Freire, Influence of Neodymium Ions on Photocatalytic Activity of TiO2 Synthesized by Sol–gel and Precipitation Methods, J. Mol. Catal. A: Chem., 2011, 336, 58–63 CrossRef CAS PubMed
.
- L. Ji, Z. Wang, Z. Li and J. Liang, Preparation of Aligned Titania Nanowires with an Aligned Carbon Nanotube Composite Template, Mater. Lett., 2008, 62, 1979–1982 CrossRef CAS PubMed
.
- M. Grandcolas, J. Ye and N. Hanagata, Combination of Photocatalytic and Antibacterial Effects of Silver Oxide Loaded on Titania Nanotubes, Mater. Lett., 2011, 65, 236–239 CrossRef CAS PubMed
.
- Y. Zhang, Y. Gao, X. H. Xia, Q. R. Deng, M. L. Guo, L. Wan and G. Shao, Structural Engineering of Thin Films of Vertically Aligned TiO2 Nanorods, Mater. Lett., 2010, 64, 1614–1617 CrossRef CAS PubMed
.
- M. S. Morey, J. D. Bryan, S. Schwarz and G. D. Stucky, Pore Surface Functionalization of MCM-48 Mesoporous Silica with Tungsten and Molybdenum Metal Centers: Perspectives on Catalytic Peroxide Activation, Chem. Mater., 2000, 12, 3435–3444 CrossRef CAS
.
- K. Okada, A. Shimai, T. Takei, S. Hayashi, A. Yasumori and K. J. D. MacKenzie, Preparation of Microporous Silica From Metakaolinite by Selective Leaching Method, Microporous Mesoporous Mater., 1998, 21, 289–296 CrossRef CAS
.
- J. K. Cochran, Ceramic Hollow Spheres and their Applications, Curr. Opin. Solid State Mater. Sci., 1998, 3, 474–479 CrossRef CAS
.
- A. Syoufian, Y. Inoue, M. Yada and K. Nakashima, Preparation of Submicrometer-Sized Titania Hollow Spheres by Templating Sulfonated Polystyrene Latex Particles, Mater. Lett., 2007, 61, 1572–1575 CrossRef CAS PubMed
.
- L. Cui, Y. Wang, M. Niu, G. Chen and Y. Cheng, Synthesis and visible light photocatalysis of Fe-doped TiO2 mesoporous layers deposited on hollow glass microbeads, J. Solid State Chem., 2009, 182, 2785–2790 CrossRef CAS PubMed
.
- G. Ma, R. Jia, J. Zhao, Z. Wang, C. Song, S. Jia and Z. Zhu, Nitrogen-Doped Hollow Carbon Nanoparticles with Excellent Oxygen
Reduction Performances and their Electrocatalytic Kinetics, J. Phys. Chem. C, 2011, 115, 25148–25154 CAS
.
- C. Wang, Y. Ao, P. Wang, J. Hou and J. Qian, Preparation, Characterization and Photocatalytic Activity of the Neodymium-Doped TiO2 Hollow Spheres, J. Appl. Surf. Sci., 2010, 257, 227–231 CrossRef CAS PubMed
.
- C. Wang, Y. Ao, P. Wang, J. Hou and J. Qian, Preparation of Cerium and Nitrogen Co-Doped Titania Hollow Spheres with Enhanced Visible Light Photocatalytic Performance, Powder Technol., 2011, 210, 203–207 CrossRef CAS PubMed
.
- P. Wang, J. Wu, Y. Ao, C. Wang, J. Hou and J. Qian, Preparation and Enhanced Photocatalytic Performance of Sn Ion Modified Titania Hollow Spheres, Mater. Lett., 2011, 65, 3278–3280 CrossRef CAS PubMed
.
- J. Xu, M. Chen and D. Fu, Study on Highly Visible Light Active Bi-Doped TiO2 Composite Hollow Sphere, Appl. Surf. Sci., 2011, 257, 7381–7386 CrossRef CAS PubMed
.
- G. An, C. Yang, S. Jin, G. Chen and X. Zhao, Hollow TiO2:Sm3+ Spheres with Enhanced Photoluminescence Fabricated by a Facile Method using Polystyrene as Template, J. Mater. Sci., 2013, 48, 5483–5488 CrossRef CAS
.
- R. G. Chaudhuri and S. Paria, Visible Light Induced Photocatalytic Activity of Sulfur Doped Hollow TiO2 Nanoparticles, Synthesized Via a Novel Route, Dalton Trans., 2014, 5526–5534 RSC
.
- K. Awazu, M. Fujimaki, C. Rockstuhl, J. Tominaga, H. Murakami, Y. Ohki, N. Yoshida and T. Watanabe, A Plasmonic Photocatalyst Consisting of Silver Nanoparticles Embedded in Titanium Dioxide, J. Am. Chem. Soc., 2008, 130, 1676–1680 CrossRef CAS PubMed
.
- Y. Tian and T. Tatsuma, Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles, J. Am. Chem. Soc., 2005, 127, 7632–7637 CrossRef CAS PubMed
.
- I. Sondi and B. Salopek-Sondi, Silver Nanoparticles as Antimicrobial Agent: a Case Study on E. coli as a Model for Gram-negative Bacteria, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS PubMed
.
- A. B. Smetana, K. J. Klabunde, G. R. Marchin and C. M. Sorensen, Biocidal Activity of Nanocrystalline Silver Powders and Particles, Langmuir, 2008, 24, 7457–7464 CrossRef CAS PubMed
.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. J. Alvarez, Antimicrobial Nanomaterials for Water Disinfection and Microbial Control: Potential Applications and Implications, Water Res., 2008, 42, 4591–4602 CrossRef CAS PubMed
.
- L. Zhang, J. C. Yu, H. Y. Yip, Q. Li and K. W. Kwong, Ambient Light Reduction Strategy to Synthesize Silver Nanoparticles and Silver-Coated TiO2 with Enhanced Photocatalytic and Bactericidal Activities, Langmuir, 2003, 19, 10372–10380 CrossRef CAS
.
- D. Guin, S. V. Manorama, J. N. L. Latha and S. Singh, Photoreduction of Silver on Bare and Colloidal TiO2 Nanoparticles/Nanotubes: Synthesis, Characterization, and Tested for Antibacterial Outcome, J. Phys. Chem. C, 2007, 111, 13393–13397 CAS
.
- Q. Xiang, J. Yu, B. Cheng and H. C. Ong, Microwave-Hydrothermal Preparation and Visible-Light Photoactivity of Plasmonic Photocatalyst Ag–TiO2 Nanocomposite Hollow Spheres, Chem.–Asian J., 2010, 5, 1466–1474 CAS
.
- C. Song, D. Wang, G. Gu, Y. Lin, J. Yang, L. Chen, X. Fu and Z. Hu, Preparation and Characterization of Silver/TiO2 Composite Hollow Spheres, J. Colloid Interface Sci., 2004, 272, 340–344 CrossRef CAS PubMed
.
- P. L. Ji, X. Z. Kong, J. G. Wang and X. L. Zhu, Characterization and Photocatalytic Properties of Silver and Silver Chloride Doped TiO2 Hollow Nanoparticles, Chin. Chem. Lett., 2012, 23, 1399–1402 CrossRef CAS PubMed
.
- P. Piccinini, C. Minero, M. Vincenti and E. Pelizzetti, Photocatalytic Mineralization of Nitrogen-Containing Benzene Derivatives, Catal. Today, 1997, 39, 187–195 CrossRef CAS
.
- D. S. Bhatkhande, V. G. Pangarkar and A. A. C. M. Beenackers, Photocatalytic Degradation of Nitrobenzene using Titanium Dioxide and Concentrated Solar Radiation: Chemical Effects and Scaleup, Water Res., 2003, 37, 1223–1230 CrossRef CAS
.
- G. M. S. ElShafei, F. Z. Yehia, O. I. H. Dimitry, A. M. Badawi and G. Eshaq, Degradation of Nitrobenzene at Near Neutral pH using Fe2+–Glutamate Complex as a Homogeneous Fenton Catalyst, Appl. Catal., B, 2010, 99, 242–247 CrossRef CAS PubMed
.
- O. A. O'connor and L. Y. Young, Toxicity and Anaerobic Biodegradability of Substituted Phenols under Methanogenic Conditions, Environ. Toxicol. Chem., 1989, 8, 853–862 CrossRef PubMed
.
- L. Zhao, J. Ma, Z.-Z. Sun and X.-D. Zhai, Catalytic Ozonation for the Degradation of Nitrobenzene in Aqueous Solution by Ceramic Honeycomb-Supported Manganese, Appl. Catal., B, 2008, 83, 256–264 CrossRef CAS PubMed
.
- E. S. Elmolla and M. Chaudhuri, Degradation of the Antibiotics Amoxicillin, Ampicillin and Cloxacillin in Aqueous Solution by the Photo-Fenton Process, J. Hazard. Mater., 2009, 172, 1476–1481 CrossRef CAS PubMed
.
- M. Rysz and P. J. J. Alvarez, Amplification and Attenuation of Tetracycline Resistance in Soil Bacteria: Aquifer Column Experiments, Water Res., 2004, 38, 3705–3712 CrossRef CAS PubMed
.
- M. A. Gilliver, M. Bennett, M. Begon, S. M. Hazel and C. A. Hart, Enterobacteria: Antibiotic Resistance Found in Wild Rodents, Nature, 1999, 401, 233–234 CrossRef CAS PubMed
.
- M. Goni-Urriza, M. Capdepuy, C. Arpin, N. Raymond, P. Caumette and C. Quentin, Impact of an Urban Effluent on Antibiotic Resistance of Riverine Enterobacteriaceae and Aeromonas spp., Appl. Environ. Microbiol., 2000, 66, 125–132 CrossRef CAS
.
- M. Muruganandham, N. Shobana and M. Swaminathan, Optimization of Solar Photocatalytic Degradation Conditions of Reactive Yellow 14 Azo Dye in Aqueous TiO2, J. Mol. Catal. A: Chem., 2006, 246, 154–161 CrossRef CAS PubMed
.
- H. Chun and W. Yizhong, Decolorization and Biodegradability of Photocatalytic Treated Azo Dyes and Wool Textile Wastewater, Chemosphere, 1999, 39, 2107–2115 CrossRef
.
- H. Guo, D. Tian, L. Liu, Y. Wang, Y. Guo and X. Yang, Core–shell TiO2 microsphere with enhanced photocatalytic activity and improved lithium storage, J. Solid State Chem., 2013, 201, 137–143 CrossRef CAS PubMed
.
- N. S. Karan, A. Agrawal, P. K. Pandey, P. Smitha, S. J. Sharma, D. P. Mishra and N. S. Gajbhiye, Diffusion flame synthesis of hollow, anatase TiO2 nanoparticles, Mater. Sci. Eng., B, 2009, 163, 128–133 CrossRef CAS PubMed
.
- X. Li, K. Lv, K. Deng, J. Tang, R. Su, J. Sun and L. Chen, Synthesis and characterization of ZnO and TiO2 hollow spheres with enhanced photoreactivity, Mater. Sci. Eng., B, 2009, 158, 40–47 CrossRef CAS PubMed
.
- C. Song, W. Yu, B. Zhao, H. Zhang, C. Tang, K. Sun, X. Wu, L. Dong and Y. Chen, Efficient fabrication and photocatalytic properties of TiO2 hollow spheres, Catal. Commun., 2009, 10, 650–654 CrossRef CAS PubMed
.
- F. Zhang, Y. Zhang, S. Song and H. Zhang, Superior electrode performance of mesoporous hollow TiO2 microspheres through efficient hierarchical nanostructures, J. Power Sources, 2011, 196, 8618–8624 CrossRef CAS PubMed
.
- M. Ray and S. Paria, Growth Kinetics of Silver Bromide Nanoparticles in Aqueous Nonionic Surfactant Solutions, Ind. Eng. Chem. Res., 2011, 50, 11601–11607 CrossRef CAS
.
- D. B. Hamal and K. J. Klabunde, Synthesis, Characterization, and Visible Light Activity of New Nanoparticle Photocatalysts Based on Silver, Carbon, and Sulfur-doped TiO2, J. Colloid Interface Sci., 2007, 311, 514–522 CrossRef CAS PubMed
.
- G. Kawamura, S. Sato, H. Muto, M. Sakai, P. B. Lim, K. Watanabe, M. Inoue and A. Matsuda, AgBr Nanocrystal-Dispersed Silsesquioxane–Titania hybrid Films for Holographic Materials, Mater. Lett., 2010, 64, 2648–2651 CrossRef CAS PubMed
.
- M. A. Behnajady, H. Eskandarloo, N. Modirshahla and M. Shokri, Investigation of the Effect of Sol–gel Synthesis Variables on Structural and Photocatalytic Properties of TiO2 Nanoparticles, Desalination, 2011, 278, 10–17 CrossRef CAS PubMed
.
- H. Zhang, G. Wang, D. Chen, X. Lv and J. Li, Tuning Photoelectrochemical Performances of Ag–TiO2 Nanocomposites via Reduction/Oxidation of Ag, Chem. Mater., 2008, 20, 6543–6549 CrossRef CAS
.
- J. Du, J. Zhang, Z. Liu, B. Han, T. Jiang and Y. Huang, Controlled Synthesis of Ag/TiO2 Core–Shell Nanowires with Smooth and Bristled Surfaces via a One-Step Solution Route, Langmuir, 2006, 22, 1307–1312 CrossRef CAS PubMed
.
- S. S. Boxi and S. Paria, Effect of Silver Doping on TiO2, CdS, and ZnS Nanoparticles for the Photocatalytic Degradation of Metronidazole under Visible Light, RSC Adv., 2014, 4, 37752–37760 RSC
.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemannt, Environmental Applications of Semiconductor Photocatalysis, Chem. Rev., 1995, 95, 69–96 CrossRef CAS
.
- W. Choi, A. Termin and M. R. Hoffmann, The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics, J. Phys. Chem., 1994, 98, 13669–13679 CrossRef
.
- J. Liqiang, F. Honggang, W. Baiqi, W. Dejun, X. Baifu, L. Shudan and S. Jiazhong, Effects of Sn Dopant on the Photoinduced Charge Property and Photocatalytic Activity of TiO2 Nanoparticles, Appl. Catal., B, 2006, 62, 282–291 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03421c |
| This journal is © The Royal Society of Chemistry 2015 |