Effect of organically modified Ni–Al layered double hydroxide loading on the thermal and morphological properties of L-methionine containing poly(amide-imide) nanocomposites
Shadpour Mallakpour*abc and
Mohammad Dinariab
aOrganic Polymer Chemistry Research Laboratory, Department of Chemistry, Isfahan University of Technology, Isfahan, 84156-83111, I. R. Iran. E-mail: mallak@cc.iut.ac.ir; mallak777@yahoo.com; mallakpour84@alumni.ufl.edu; Fax: +98-31-3391-2350; Tel: +98-31-3391-3267
bNanotechnology and Advanced Materials Institute, Isfahan University of Technology, Isfahan, 84156-83111, I. R. Iran
cCenter of Excellence in Sensors and Green Chemistry, Department of Chemistry, Isfahan University of Technology, Isfahan, 84156-83111, I. R. Iran
First published on 13th March 2015
Abstract
This work deals with the synthesis of an organically modified Ni–Al layered double hydroxide (LDH) and the effect of the LDH loading on the thermal and morphological behavior of poly(amide-imide)/Ni–Al LDH nanocomposites. Modified Ni–Al LDH was synthesized by a co-precipitation reaction of Ni(NO3)2·6H2O, Al(NO3)3·9H2O and N,N′-(pyromellitoyl)-bis-L-methionine under ultrasonic irradiation in a short time. The X-ray diffraction (XRD) analysis reveals that the d-spacing value of the modified LDH increases to 2.00 nm from 0.76 nm for the unmodified LDH, which confirms the insertion of a diacid molecule into the LDH interlayers. L-Methionine containing poly(amide-imide) blends with various modified-LDH content (2, 4 and 8 wt% with respect to polymer) were prepared in ethanol under sonication. The structural properties and the thermal behavior of the modified-LDH and polymer based nanocomposites were characterized by XRD, field emission-scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR) spectra and thermogravimetry analysis (TGA) techniques. TEM and FE-SEM results revealed the exfoliated modified-LDH in the polymer matrix. According to the TGA results, the prepared nanocomposites show significantly improved thermal stability at higher temperature because of the homogeneous and good dispersion of modified LDH in the polymeric matrix.
1. Introduction
Layered double hydroxides (LDHs) are a class of anionic clay that consist of positively charged metal hydroxide sheets and anions in the interlayer to counterbalance the charges.1–3 They can be represented by the general formula [M1−x2+Mx3+(OH)2]x+(Ax/nn−)·yH2O, where M2+ and M3+ are divalent and trivalent metallic cations, respectively, An− represents intercalated anions, such as Cl−, CO32− or NO3−, and x is the stoichiometric coefficient of M2+ and M3+ in the range of 0.20 to 0.40. The type of metallic ions and the charge-balancing anions together with the value of x can be diverse over a wide range, giving rise to a large class of iso-structural materials.4–8 These materials exhibit attractive physical and chemical properties such as exceptional absorptivity, anion exchange capacity, high chemical inertness, high physical and chemical stability and low production costs.9,10 Other important properties of these anionic clays are their easy synthesis, no toxicity and “memory effect”.11 LDHs were used in different areas for instance medical materials, gene delivery, catalysts, and polymer nanocomposites.12–16Because of their potential to significantly improve the flame retardancy, LDH were used in the polymer based nanocomposites (NCs).13,17–19 On the other hand, owing to the strong hydrophilicity, high surface-to-volume ratio, and strong affinity between the two hydroxide layers of LDH, homogeneous dispersion of these compounds within the polymer matrices is difficult and they should be modified before using a as nanofiller.19 One of the common organic modifier which was used for LDH's modification is sodium dodecylbenzenesulfonate (SDBS), that can increased the interlayer distance and make the layers more organophillic. However, it is flammable and furthermore, it is quite inert, meaning that only the van der Waals forces and hydrogen bonding exist between SDBS intercalated LDH and the polymer matrices.20,21 It may cause a limited enhancement on the mechanical properties of the resulting materials. So, novel functional LDH's modifier with low flammability would be significant for developing high performance NC materials based on polymer and LDH nanofillers.21
It has been reported that polymeric based NCs exhibit unique properties including improved thermo-mechanical properties, gas barrier performance, improved thermal properties, and greatly reduced.22 Recently, polycondensation polymers have fascinated attractions in nanocomposite production.22–24 Poly(amide-imide)s (PAI)s, as an important type of the high performance materials, have been developed as special division of macromolecules that offer a concession between the superior mechanical properties associated with amide units and the high thermal stability by imide structures.25–27 The incorporation of natural amino acids in the backbone of the synthetic PAIs can lead to optically active polymers with increased solubility, biocompatibility and biodegradability.28,29 These type of macromolecules can be used in different area such as nonlinear optical devices, chiral medium for asymmetric synthesis, chiral phases for enantiomeric separations in chromatography methods, and etc.30,31
Modification of LDHs with carboxylate anions has attracted considerable attention in recent years.32–34 Among the organic modifiers for functionalization of LDH, amino acids as the simplest chiral biomolecules are the best candidates due to the their nontoxic, biocompatible and biodegradable properties.4,35 By using amino acid containing bolding black materials as modifiers for LDHs in comparison with common chemically synthesized modifier, amino acid bio-surfactants have the important advantage of biodegradability, low toxicity and various possible structures.36
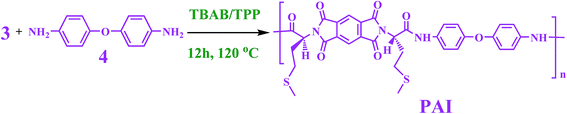
In our previous studies several NC materials were prepared by MgAl-LDH nanofiller and poly(vinyl alcohol), polyvinylpyrrolidone as well as amino acid based polymers as matrixes.19,36–41 In continues to these studies, in this paper, modified Ni–Al LDH was synthesized by co-precipitation reaction of Ni(NO3)2·6H2O, Al(NO3)3·9H2O and N,N′-(pyromellitoyl)-bis-L-methionine under ultrasonic irradiation in aqueous solution. L-Methionine containing PAI was synthesized by the direct polycondensation reaction of N,N′-(pyromellitoyl)-bis-L-methionine and 4,4′-diaminodiphenylether in molten tetrabutylammonium bromide. Then, NCs of PAI with different LDHs content were prepared in ethanol solution for the first time. The structure and morphology of the obtained materials was investigated by Fourier transfer infrared (FT-IR), X-ray diffraction (XRD), thermogravimetric analysis (TGA), field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM) techniques.
2. Experimental
2.1. Materials
L-Methionine, pyromellitic dianhydride (PMDA), nikel(ii) nitrate hexahydrate [Ni(NO3)2·6H2O], aluminium(III) nitrate nonahydrate [Al(NO3)3·9H2O], TBAB, glacial acetic acid, triphenyl phosphite (TPP), 4,4′-diaminodiphenylether and sodium hydroxide (NaOH) were purchased from Merck Chemical Co (Darmstadt, Germany) and used without further purification. N,N′-Dimethylformamide (DMF) was distilled over barium oxide under reduced pressure before use.2.2. Preparation of methionine containing diacid
N,N′-(Pyromellitoyl)-bis-L-methionine diacid was prepared according to our previous article.362.3. Preparation of diacid-modified NiAl-LDH
Diacid modified NiAl-LDH was prepared in one step under ultrasonic irradiation. Al(NO3)3·9H2O and Ni(NO3)2·6H2O were dissolved in deionized water to obtain solution 1. An aqueous solution containing NaOH (0.02 mol) and N,N′-(pyromellitoyl)-bis-L-methionine (0.02 mol) as solution 2; was prepared and stirred at R.T. Solution 2 was added to the solution 2 and the resultant suspension was sonicated for 1 h to obtained modified NiAl-LDH. Finally, the obtained precipitate was filtered and washed by deionized water and then dried at 60 °C for 24 h. For comparative study, NiAl LDH–CO32− was prepared under identical reaction conditions without using diacid compound.2.4. Preparation of PAI
Chiral PAI was prepared by direct step-growth polymerization reaction of diacid and diamine monomers a follow: into a 25 mL flask fitted with a mechanical stirrer, 0.33 mmol (0.10 g) of diacid 3, 0.33 mmol (0.08 g) of diamine 4 and 1.37 mmol (0.44 g) of TBAB were placed and the mixture was completely ground. After that, 0.78 mL of TPP was added and the reaction temperature was raised to 120 °C for 12 h. Finally it was cooled to room temperature and 20 mL of methanol was dropped into the viscous polymer solution to obtain the final polymer precipitate.2.5. Synthesis of PAI/modified-LDH NCs
At first, 0.20 g of the PAI was dispersed in 30 mL of absolute ethanol and sonication for 30 min at room temperature until uniform colloidal dispersion was obtained. Then the suspension was mixed with 2, 4 and 8 wt% of diacid modified NiAl-LDH and it was sonicated for 2 h at room temperature to produced polymer based NCs. The solvent was removed and the obtained solid was dried in vacuum at 80 °C for 6 h.2.6. Characterization
FT-IR spectra were recorded on a Jasco-680 spectrometer in 400–4000 cm−1 region using KBr pellets.X-ray diffraction (XRD) spectra were registered on a Bruker, D8 Advance X-ray diffractometer, in the range 2θ of 1.2° and 80°. An X-ray beam characteristic to CuKα radiation was used (λ = 1.5418 Å). Basal spacing were determined from the position of the d(003) reflection.
Thermogravimetric analyses (TGA) were done on STA503 TA instrument from 30 to 800 °C nitrogen atmosphere. The heating rate was 10 °C min−1.
FE-SEM images were acquired on a HITACHI; S-4160 instrument. Prior to the analysis the materials were fractured in liquid nitrogen. The fresh fractured surface was covered with a gold layer for a better contrast.
TEM images were registered on a Philips CM120 TEM microscope using an accelerator voltage of 100 kV.
MISONIX ultrasonic liquid processor, XL-2000 series with a wave of frequency 2.25 × 104 Hz and power 100 W was employed for preparing of the new materials.
3. Results and discussion
3.1. Preparation of diacid modified NiAl-LDH and polymer based NCs
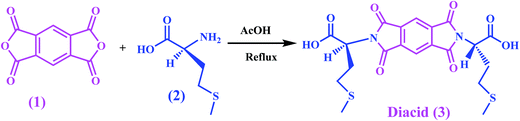
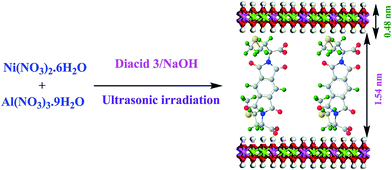
In this study, L-methionine amino acid was used as building blocks for the synthesis of diacid modified NiAl-LDH. Diacid 3 was synthesized from the reaction of PMDA and methionine in refluxing acetic acid solution as shown in Scheme 1. Then, this compound was used for the preparation of new modified-LDH under ultrasonic irradiation in an aqueous solution. The spatial orientation of the diacid modified NiAl-LDH is shown in Fig. 1. According to the Fig. 1, the anion of dicarboxylate was considered to be arranged vertically to the LDH basal layer.L-Methionine containing PAI was prepared in the TBAB/TPP system as condensing agent and green reaction media by the direct polymerization reaction of chiral diacid 3 with 4,4′-diaminodiphenylether 4 as shown in Scheme 2. The inherent viscosity of the PAI under optimized condensations was 0.38 dL g−1 and yield was 94%.29 This chiral polymer was used as matrix for the preparation of PAI/modified-LDH NCs.
3.2. Characterization
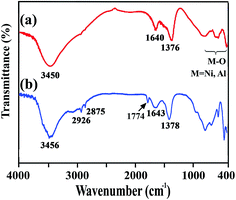
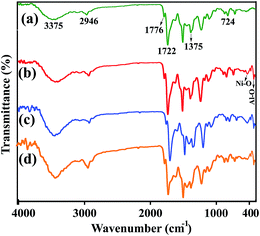
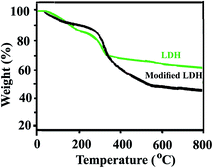
The FT-IR spectra of the neat PAI and PAI NCs with different amount of modified-LDH are presented in Fig. 3. The FT-IR spectrum of chiral PAI showed absorptions of N–H amide bonds appeared around 3375 cm−1. The aliphatic C–H stretching peak was also appeared at around 2946 cm−1. The asymmetric and symmetric stretching vibrations of the imide carbonyl groups are observed at 1776 and 1722 cm−1. The bands of C–N stretching and ring deformation appeared at 1375 and 724 cm−1, respectively (Fig. 3a). In the FT-IR spectra of NCs materials with 2, 4 and 8 wt% of modified-LDH in compression with the pristine PAI, the new bands around 400–650 cm−1 are due to the lattice vibrations of the hydroxide sheets. The bands at 600, 570 and 425 cm−1 are due to Ni–O–Al deformation mode and Ni–O and Al–O stretching modes.43 Thus from the FT-IR spectroscopy, the interaction between PAI and modified LDH and their complex formation have been confirmed (Fig. 3b–d).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
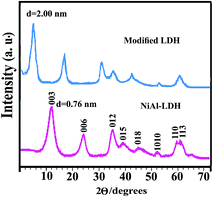
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where λ is the X-ray wavelength (1.5418 Å). This value represents the thickness of the brucite like layer as well as the size of anions and number of water molecules existing in the interlayer (Fig. 4). As expected, the position of the first order basal reflection (003) in the modified-LDH is shifted to a higher d-value, indicating an expansion in the interlayer distance (Fig. 4). In addition, the presence of reflections strongly elucidates the high crystalline order in the modified LDH material. The main diffraction peak of modified-LDH is obtained at 2θ value of 4.4° and the corresponding d003 value is 2.00 nm. The size of the diacid was anticipated and compared to the obtained basal spacing via XRD patterns. As shown in previous study, the thickness of LDH sheets is 0.48 nm.44 The gallery height between the diacid modified-NiAl LDH can be calculated as 1.52 nm. This means that the diacid molecule is vertically oriented in the interlayer space, which is confirmed by the approximate molecule length of L-methionine containing diacid which was calculated by ChemOffice as 1.56 nm (Fig. 1).
θ, where λ is the X-ray wavelength (1.5418 Å). This value represents the thickness of the brucite like layer as well as the size of anions and number of water molecules existing in the interlayer (Fig. 4). As expected, the position of the first order basal reflection (003) in the modified-LDH is shifted to a higher d-value, indicating an expansion in the interlayer distance (Fig. 4). In addition, the presence of reflections strongly elucidates the high crystalline order in the modified LDH material. The main diffraction peak of modified-LDH is obtained at 2θ value of 4.4° and the corresponding d003 value is 2.00 nm. The size of the diacid was anticipated and compared to the obtained basal spacing via XRD patterns. As shown in previous study, the thickness of LDH sheets is 0.48 nm.44 The gallery height between the diacid modified-NiAl LDH can be calculated as 1.52 nm. This means that the diacid molecule is vertically oriented in the interlayer space, which is confirmed by the approximate molecule length of L-methionine containing diacid which was calculated by ChemOffice as 1.56 nm (Fig. 1).
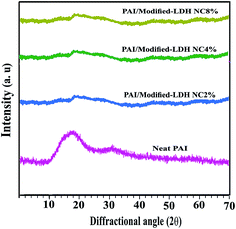
The XRD patterns of the neat PAI and NCs with 2, 4 and 8% of modified-LDH are shown in Fig. 5. For neat PAI no crystalline peak was observed, indicating that this polymer is amorphous. Amorphous phase of PAIs is favorable to its good solubility. In the XRD patterns of the NCs, the diffraction peaks corresponding to the modified-LDH were disappear (Fig. 5). This complete disappearance of LDH peaks may be due to the partial exfoliated structure, in which the gallery height of intercalated layers is large enough and the layer correlation is not detected by XRD (Fig. 5). It is clear that the diacid used for LDH modification plays an important role in achieving a good dispersion, by increasing the basal distance between the LDH layers (from 0.76 to 2.00 nm) and also by creating a more hydrophobic environment within the LDH galleries which facilitates the penetration of PAI chain inside the galleries and thus the intercalation/exfoliation process to occur. Microscopic examination is required to complete characterization of NC morphology.
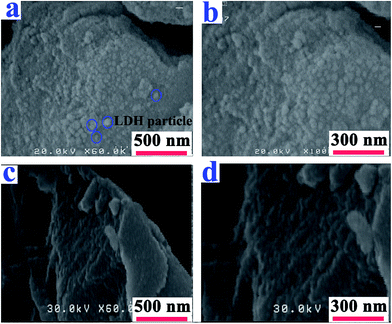
The fractured surfaces of the neat PAI and PAI NCs with 2, 4 and 8% of modified-LDH are shown in Fig. 7. According to Fig. 7b–d, the micrograph exhibits the dispersion of LDH into the polymer matrix. Some aggregation also was observed in the NC materials.
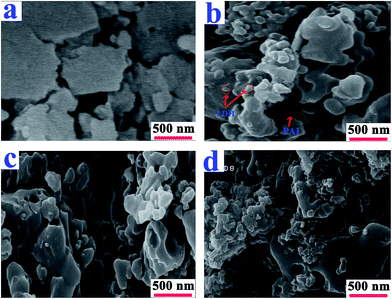 | ||
| Fig. 7 FE-SEM photographs of the (a) pure PAI and PAI NC with (b) 2%, (c) 4% and (d) 8% of modified-LDH. | ||
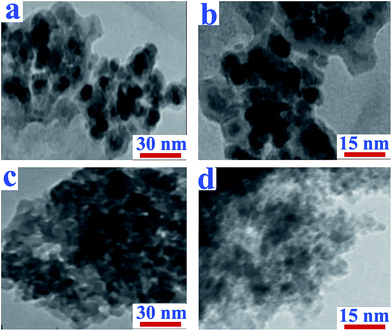
TEM presents an actual image of LDH platelets to permit identification of internal morphology of the resulting nanohybrids. Typical TEM images of diacid modified-LDH and NC of PAI with 4% of modified-LDH are presented in Fig. 8. For modified-LDH, most of the platelets adopt a hexagonal shape as shown in Fig. 8a and b. In the TEM images of PAI with 4 wt% of modified-LDH, the results show that the modified-LDH layers are mostly dispersed throughout the polymer matrix and the LDH layers are disorderly oriented providing an ample evidence of crystal layer delamination/exfoliation from their surfaces (Fig. 8c and d). It is interesting to mention that, according to the TEM images, the particles sizes of the LDH were decreases in the NCs in comparison with the neat modified LDH. It is evident from the data that, at first the PAI chains enter within the inter-layer region of the modified-LDH particles and then push the metal hydroxide sheets apart from each other.45 Then under ultrasonic irradiation, the number of the polymer chains which enter the inter-layer region of modified-LDH were increased and facilitated the delamination of the surface layers one by one from a primary particle. Therefore, in the resulting NCs, the exfoliated particle fragments are formed and the size of the original primary particles are reduced.45 According to this results, the average size of the primary particles observed in PAI NC with 4% of modified-LDH is smaller than that observed in case of modified-LDH prior to ultrasonic irradiation.
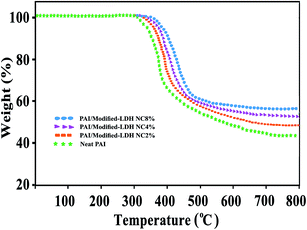
The thermal properties of the PAI and the PAI NCs with different amount of modified-LDH were estimated by means of TGA as shown in Fig. 10. Table 1 show the data for the thermal degradation of the PAI and PAI/modified NiAl-LDH NCs, including the temperature at which 5% (T5), 10% degradation occurs (T10) and char yield at 800 °C. Neat PAI show good thermal stability due to the existence of various linkages such as imide and amide groups in polymer backbones. The onset of decomposition temperature of the NCs was higher than that of pure PAI, shifting toward higher temperatures as the amount of modified-LDH was increased (Table 1). The increase in thermal stability of PAI matrix upon incorporation of modified-LDH could be attributed to the higher heat transfer capacity of modified-LDH that facilitated heat dissipation within the composites, thus preventing the accumulation of heat at certain points for degradation.42 The char yield of hybrid NCs are higher than that of pure PAI. The residual weight for these NCs at 800 °C ranged from 49% to 57%, and for pure PAI it is 45%. These results show that the thermal stability of the hybrid NCs is enhanced by the incorporation of the diacid modified NiAl-LDH. The increase of the thermostability at high degradation stages is due to the hindering effect of LDH layers which can trap the volatile gases. Also, the water from LDH galleries released during the burning process may exhibit a cooling effect on the materials.46 The thermal properties of these NCs materials were compared with our previous article based on N,N′-(pyromellitoyl)-bis-valin and 3,5-diamino-N-(thiazole-2-yl)benzamide,38 and the results demonstrated that the resulting NCs in this study have a better thermal stability then those obtained by the polymer with thiazole side groups.
| Sample | Decomposition temperature (°C) | Char yieldb (%) | LOIc | |
|---|---|---|---|---|
| T5a | T10a | |||
| a Temperature at which 5 and 10% weight loss was recorded by TGA at heating rate of 10 °C min−1 in a N2 atmosphere.b Weight percent of the material left under composed after TGA at maximum temperature 800 °C in a N2 atmosphere.c Limiting oxygen index (LOI) evaluating at char yield at 800 °C. | ||||
| PAI/LDH | 338 | 354 | 45 | 35.5 |
| PAI/LDH NC 2% | 353 | 370 | 49 | 37.1 |
| PAI/LDH NC 4% | 381 | 392 | 53 | 38.7 |
| PAI/LDH NC 8% | 394 | 408 | 57 | 40.3 |
Limiting oxygen index (LOI) of the polymer and NC materials were calculated based on their char yield at 800 °C according to the Van Krevelen and Hoftyzer equation.47
| LOI = 17.5 + 0.4CR |
The PAI/modified NiAl-LDH NCs have LOI values above 37. On the basis of LOI values, the more amounts of modified NiAl-LDH, the more LOI values and, therefore, these PAI/modified-LDH NCs can be classified as self extinguishing NC materials (Table 1).
4. Conclusions
Organically modified Ni–Al LDH was synthesized via co-precipitation method and the influences of LDH loading on the thermal and morphological behavior of PAI/modified-LDH NCs were successfully investigated. The XRD patterns of the modified NiAl-LDH show that the diacid is intercalated in the interlayer region of LDH and enlarge the interlayer distance. Then an optically active amino acid containing PAI with good solubility was selected as a matrix for the preparation of PAI based NC with different amount of modified-LDH by simple ultrasonic method in ethanol. TEM and XRD results show that the modified-LDH layers are mostly dispersed throughout the polymer matrix and the layers are disorderly oriented providing an ample evidence of crystal layer delamination/exfoliation from their surfaces. Also, according to the TEM images, the average size of the primary particles observed in PAI NC with 4% of modified-LDH is smaller than that observed in case of modified-LDH. TGA data show that the incorporation of modified-LDH increases the thermal stability of the resulting NCs. The increase of the thermostability at high degradation stages is due to the hindering effect of LDH layers which can trap the volatile gases. The presence of amino acids in modified-LDH and PAI makes synthesized NCs as biodegradable materials and suitable for industrial application.Acknowledgements
This work was supported by the Research Affairs Division Isfahan University of Technology (IUT), Isfahan. The authors are thankful to National Elite Foundation (NEF) and Iran Nanotechnology Initiative Council (INIC) for further financial support.References
- J. A. Gursky, S. D. Blough, C. Luna, C. Gomez, A. N. Luevano and E. A. Gardner, J. Am. Chem. Soc., 2006, 128, 8376–8377 CrossRef CAS PubMed.
- G. R. Williams and D. O'Hare, J. Mater. Chem., 2006, 16, 3065–3074 RSC.
- S. He, Z. An, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2013, 49, 5912–5920 RSC.
- N. T. Whilton, P. J. Vickers and S. Mann, J. Mater. Chem., 1997, 7, 1623–1629 RSC.
- F. Li and X. Duan, Struct. Bonding, 2006, 119, 193–223 CrossRef CAS.
- L. Desigaux, M. B. Belkacem, P. Richard, J. Cellier, P. Leone, L. Cario, F. Leroux, C. Taviot-Gueho and B. Pitard, Nano Lett., 2006, 6, 199–204 CrossRef CAS PubMed.
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS PubMed.
- L. Li, W. Gu, J. Chen, W. Chen and Z. P. Xu, Biomaterials, 2014, 35, 3331–3339 CrossRef CAS PubMed.
- D. G. Evans and X. Duan, Chem. Commun., 2006, 6, 485–496 RSC.
- Z. Zhu, L. Qu, Y. Guo, Y. Zeng, W. Sun and X. Huang, Sens. Actuators, B, 2010, 151, 146–152 CrossRef CAS PubMed.
- C. Andronescu, S. A. Garea, E. Vasile and H. Iovu, Compos. Sci. Technol., 2014, 95, 29–37 CrossRef CAS PubMed.
- D. G. Evans and X. Duan, Chem. Commun., 2006, 485–496 RSC.
- F. R. Costa, M. Saphiannikova, U. Wagenknecht and G. Heinrich, Adv. Polym. Sci., 2008, 210, 101–168 CrossRef CAS.
- P. Gunawan and R. Xu, J. Pharm. Sci., 2008, 97, 4367–4378 CrossRef CAS PubMed.
- Y. Zhang, B. Cui, C. Zhao, H. Lin and J. Li, Phys. Chem. Chem. Phys., 2013, 15, 7363–7369 RSC.
- L. Chen, B. Sun, X. Wang, F. Qiao and S. Ai, J. Mater. Chem. B, 2013, 1, 2268–2274 RSC.
- S. V. Krishna and G. Pugazhenthi, J. Exp. Nanosci., 2013, 8, 19–32 CrossRef CAS.
- B. Pradhan and S. K. Srivastava, Composites, Part A, 2014, 56, 290–299 CrossRef CAS PubMed.
- S. Mallakpour and M. Dinari, Polymer, 2013, 54, 2907–2916 CrossRef CAS PubMed.
- W. D. Lee, S. S. Im, H. M. Lim and K. J. Kim, Polymer, 2006, 47, 1364–1371 CrossRef CAS PubMed.
- N. J. Kang and D. Y. Wang, J. Mater. Chem. A, 2013, 1, 11376–11383 CAS.
- H. L. Tyan, C. M. Leu and K. H. Wei, Chem. Mater., 2001, 13, 222–226 CrossRef CAS.
- H. B. Hsueh and C. Y. Chen, Polymer, 2003, 44, 1151–1161 CrossRef CAS.
- F. Grillard, P. Poulin, A. Korzhenko, P. Gaillard and C. Zakri, ACS Macro Lett., 2014, 3, 224–228 CrossRef CAS.
- S. Mallakpour and M. Dinari, Amino Acids, 2012, 43, 1605–1613 CrossRef CAS PubMed.
- S. Agrawal and A. K. Narula, Polym. Bull., 2013, 70, 3241–3260 CrossRef CAS PubMed.
- J. M. Dodda, T. Kovarik, J. Kadlec and L. Kullova, Polym. Degrad. Stab., 2013, 98, 2306–2316 CrossRef CAS PubMed.
- F. Sanda and T. Endo, Macromol. Chem. Phys., 1999, 200, 2651–2661 CrossRef CAS.
- S. Mallakpour and M. Dinari, J. Macromol. Sci., Part A: Pure Appl.Chem., 2011, 48, 644–679 CrossRef CAS.
- K. Soai and S. Niwa, Chem. Rev., 1992, 92, 833–856 CrossRef CAS.
- C. L. Levesque, S. Moehn, P. B. Pencharz and R. O. Ball, Livest. Sci., 2010, 133, 4–9 CrossRef PubMed.
- S. Carlino, Solid State Ionics, 1997, 98, 73–84 CrossRef CAS.
- G. G. C. Arizag, A. S. Mangrich, J. E. F. da Costa Gardolinski and F. Wypych, J. Colloid Interface Sci., 2008, 320, 168–176 CrossRef PubMed.
- C. Nyambo, P. Songtipya, E. Manias, M. M. Jimenez-Gasco and C. A. Wilkie, J. Mater. Chem., 2008, 18, 4827–4838 RSC.
- S. Aisawa, S. Takahashi, W. Ogasawara, Y. Umetsu and E. Narita, J. Solid State Chem., 2001, 162, 52–62 CrossRef CAS.
- S. Mallakpour, M. Dinari and V. Behranvand, RSC Adv., 2013, 3, 23303–23308 RSC.
- S. Mallakpour and M. Dinari, Polym.-Plast. Technol. Eng., 2014, 53, 880–889 CrossRef CAS.
- S. Mallakpour and M. Dinari, Polym.-Plast. Technol. Eng., 2014, 53, 1047–1055 CrossRef CAS.
- S. Mallakpour and M. Dinari, Prog. Org. Coat., 2014, 77, 583–589 CrossRef CAS PubMed.
- S. Mallakpour and M. Dinari, J. Polym. Res., 2014, 21, 350–358 CrossRef.
- S. Mallakpour, M. Dinari and A. Nabiyan, J. Polym. Res., 2015, 22, 11–19 CrossRef.
- J. T. Kloprogge, L. Hickey and R. L. Frost, J. Raman Spectrosc., 2004, 35, 967–974 CrossRef CAS.
- B. Du and Z. Fang, Nanotechnology, 2010, 21, 315603–315608 CrossRef PubMed.
- O. C. Wilson, T. Olorunyolemi, A. Jaworski, L. Borum, D. Young, A. Siriwat, E. Dickens, C. Oriakhi and M. Lerner, Appl. Clay Sci., 1999, 15, 265–279 CrossRef CAS.
- F. R. Costa, U. Wagenknecht and G. Heinrich, Polym. Degrad. Stab., 2007, 92, 1813–1823 CrossRef CAS PubMed.
- M. Ardanuy and J. I. Velasco, Appl. Clay Sci., 2011, 51, 341–347 CrossRef CAS PubMed.
- D. W. Van Krevelen and P. J. Hoftyzer, Properties of polymers, Elsevier scientific publishing, New York, 3rd edn, 1976 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |