Activation of C–H bonds of thiosemicarbazones by transition metals: synthesis, structures and importance of cyclometallated compounds
Abstract
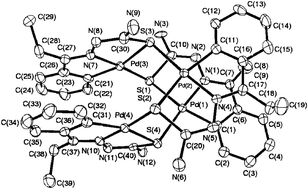
Transition metals, namely, palladium(II), platinum(II), ruthenium(II), rhodium(III) and iridium(III) have induced activation of C–H bonds of thiosemicarbazones and yielded mono-, di-, tri- and tetra-nuclear complexes with or without tertiary phosphines as co-ligands. Mono-thiosemicarbazones (R1R2C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N3–N2H–C1(
N3–N2H–C1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)–N1R3R4) undergo loss of C–H (R1 ring) and N2–H protons and formed cyclometallated derivatives. In mono- and di-nuclear complexes, the thio-ligands coordinate as terdentate (C,N,S) dianions, while in tri- and tetra-nuclear complexes, these ligands act as tetradentate (C,N,μ-S) dianions. The mono-thiosemicarbazones generally involve μ-S bridging in oligomers. Only one bis-thiosemicarbazone is reported to form mononuclear or mixed metal di-nuclear complexes. This review describes synthetic aspects, molecular structures, electronic absorption spectroscopy, fluorescence, cyclic voltammetry, NMR(1H, 13C, 31P) spectroscopy and applications (biological, catalysis) of complexes. The factors for metallation and conclusions with future scope of investigations are also mentioned. Literature coverage is upto date, to the best of my knowledge, though a few omissions cannot be ruled out.
S)–N1R3R4) undergo loss of C–H (R1 ring) and N2–H protons and formed cyclometallated derivatives. In mono- and di-nuclear complexes, the thio-ligands coordinate as terdentate (C,N,S) dianions, while in tri- and tetra-nuclear complexes, these ligands act as tetradentate (C,N,μ-S) dianions. The mono-thiosemicarbazones generally involve μ-S bridging in oligomers. Only one bis-thiosemicarbazone is reported to form mononuclear or mixed metal di-nuclear complexes. This review describes synthetic aspects, molecular structures, electronic absorption spectroscopy, fluorescence, cyclic voltammetry, NMR(1H, 13C, 31P) spectroscopy and applications (biological, catalysis) of complexes. The factors for metallation and conclusions with future scope of investigations are also mentioned. Literature coverage is upto date, to the best of my knowledge, though a few omissions cannot be ruled out.

 Please wait while we load your content...
Please wait while we load your content...