Encrustation of cobalt doped copper ferrite nanoparticles on solid scaffold CNTs and their comparison with corresponding ferrite nanoparticles: a study of structural, optical, magnetic and photo catalytic properties†
Abstract
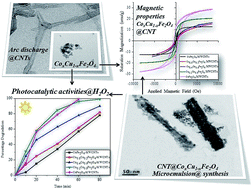
Cobalt doped copper ferrite nanoparticles and their nanocomposites with carbon nanotubes (CNTs) were synthesized by microemulsion method where sodium dodecyl sulphate was used as soft templating agent to control the particle size and shape. Powder X-ray diffraction technique confirmed the formation of cobalt doped copper ferrite nanoparticles and their corresponding nanocomposites. A significant decrease in the lattice parameter was observed using Le-bail refinement method which confirmed the doping of cobalt ion in to the copper ferrite lattice. Appearance of peak at around 25.9° indicated that CNTs remain unaffected during synthesis procedure. High Resolution Transmission Electron Microscopy (HR-TEM) was used to estimate the particle size, shape and uniformity of all the synthesized samples. Particle size was observed to be around 4–5 nm. A fine layer of ferrite nanoparticles on the surface of CNTs was also confirmed. Optical studies of the entire samples showed wide coverage of visible light spectrum from 675–1050 nm. Magnetic studies of the all samples were carried out using vibrating sample magnetometer and a significant increase in the saturation magnetization and coercivity values with cobalt ion doping was observed. This increase could be attributed to the higher magnetic moment of Co2+ ions (3 μB) as compared to Cu2+ ion (1 μB) at B-sub lattice. Comparative photocatalytic activity was also elevated and it was found that ferrite–CNTs nanocomposites show higher catalytic activities for the degradation of Rhodamine B dye in comparison with the ferrite nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...