Electrical charge-induced selective ion permeation in HfO2/porous nickel silicide hierarchical structures†
Chih-Chung Lai‡
a,
Chung-Han Lu‡a,
Chia-Kai Linb,
Hsuan-Chu Chena,
Fan-Gang Tsengbc and
Yu-Lun Chueh*a
aDepartment of Materials Science and Engineering, National Tsing Hua University, Hsinchu, 30013, Taiwan, Republic of China. E-mail: ylchueh@mx.nthu.edu.tw
bDepartment of Engineering and System Science, National Tsing Hua University, Hsinchu, 30013, Taiwan, Republic of China
cDivision of Mechanics, Research Center for Applied Sciences, Academia Sinica, Taiwan, Republic of China
First published on 24th April 2015
Abstract
HfO2/porous nickel silicide (NiSi) hierarchical structures fabricated by metal-assisted chemical etching (MACE) followed by a silicidation process and deposition of HfO2 by atomic layer deposition (ALD) have been. The as-formed porous structures are systematically investigated using etching solutions of various HF/H2O2 ratios and the manipulation of electrical double-layer (EDL) overlapping, leading to electrical charge-induced selective ion penetration in HfO2/porous NiSi hierarchical structures. Its desalination ability was examined, in which the ionic concentration of saline water can be reduced by 21% when a bias of 2 V was applied. In addition, the mechanical property of the as-formed porous NiSi membrane was estimated to have a flexural strength of 124.04 MPa. These porous NiSi membranes can be continuously operated for 18 h without loss of its deionization ability.
Introduction
Increasing demand for water resources has attracted global attention due to industrialization and rapid population growth.1 It is well known that the ocean contains abundant water resources for human beings. Therefore, the question of how to effectively utilize seawater desalination, which is a promising method for solving freshwater shortages, is one of the most difficult challenges that we have to face nowadays.Desalination technologies, including multi-stage flash (MSF), multi-effect distillation (MED), reverse osmosis (RO) and electro-dialysis (ED) have been developed.2–4 For MSF and MED, thermally heating seawater into pure water vapour, followed by condensation into pure water is a power-consuming process. An operating pressure of ∼40–82 bar is needed to overcome osmotic pressure (∼27 bar for seawater) through an RO semi-permeable membrane with a pore size of ∼1 nm, leading to greater power consumption. The utilization of an additional electrical field for the selective separation of ions, leaving pure water through perm-selective membranes, is a typical feature of the ED process, whereas the extra pressure is still unavoidable as a driving force to concentrate the salt solution.
Currently, the total global desalination capacity is of ∼80 million m3 per day and is expected to reach ∼100 million m3 per day by 2015.5 Despite the availability of seawater resources, sustainable freshwater supplies through desalination can only be realized by developing new technologies, which can address the challenge of significantly reducing its energy consumption. For example, RO is the most energy-efficient technique (average power consumption of 2–5 kWh m−3 has been recently achieved). The key factor for reducing energy consumption relies on improving energy efficiency, pushing the water through the membrane. However, the simultaneous maintenance of the efficient performance of both water permeability and water/salt selectivity for RO membranes is difficult.6 Recently, a new desalination process that utilizes ion concentration polarization (ICP) phenomena, yielding a depletion region in a heterogeneous nanoporous junction inside the micro-/nano-channel, has been reported. In this method, diffusion of ions is restrained, allowing only water molecules penetrating through the channel to result in direct desalination of seawater.7,8 Based on this concept, we intend to develop another technical pathway using membranes with larger pore sizes.9 In fact, the desalination of saline water can be primarily demonstrated by electrical charge-induced selective ionic permeation via the manipulation of electrical double-layer (EDL) overlapping in anodic aluminum oxide (AAO) as the desalination membrane. Ideally, it represents a lower energy-consumption process, compared to MSFs and EDs, even being more energy-efficient than ROs.10 However, the brittleness of the AAO membranes make it difficult to survive because they can be easily broken during the desalination operation. In addition, the function of deionization still fails after 10 h of continuous operation. In this study, the improved membrane assembly by the fabrication of HfO2/porous NiSi hierarchical structures was achieved and its deionization ability and mechanical properties were tested and measured. The as-formed porous structures were investigated using etching solutions with various ratios of HF/H2O2, and electrical charge-induced selective ionic transport in HfO2/porous NiSi hierarchical structures with different biases was examined with which the ion concentration was reduced by 21% once the applied bias reached 2 V. Furthermore, these porous NiSi membranes can be continuously operated without the loss of its deionization ability for at least 18 h. In addition, the enhanced mechanical properties of HfO2/porous NiSi membrane were examined, which was found to be mechanically stronger than the AAO membrane we used in our previous study.10
Experimental section
Fabrication of HfO2/porous NiSi membranes
Four-inch, (001)-oriented, 10–25 Ω cm, p-type Si wafers were used as starting substrates, which were sliced into 2.5 × 2 cm2-sized pieces. These samples were cleaned using a standard cleaning process through ultrasonication for the removal of organic particles by subsequent immersion in acetone, alcohol and DI water. A thin gold film (20 nm-thick) was selectively deposited on a Si substrate by an evaporator with a deposition rate of 0.2 nm s−1, followed by annealing for 5 min at 1000 °C to obtain Au NP arrays. Au NPs/Si samples were placed in a mixed solution containing HF (49 wt%, ECHO Chemical Co.), H2O2 (30 wt%, Showa Chemical Industry Co.) and deionized water to form porous Si structures. Subsequently, both the sides of the porous Si structure were then mechanically polished to produce smoother surfaces for use in subsequent manufacturing processes.11 A 200 nm-thick Ni layer was evaporated and deposited on the porous Si substrate, followed by annealing to obtain an NiSi-conductive layer. For the growth of HfO2 thin films by atomic layer deposition (ALD) process, tetrakis(dimethylamido) hafnium [TDMA-Hf] was used as a hafnium precursor and H2O molecules were used as oxidizing agents. The deposition rate was almost 0.1 nm per cycle at a reaction temperature of 224 °C.Characterization
Surface morphologies of porous silicon structures were examined by field-emission scanning electron microscopy (FE-SEM, S-8010, Hitachi). A Shimadzu XRD-6000 spectrometer with Cu Kα monochromatic radiation sources at 40 kV and 30 mA was used to characterize microstructures and phases. An Agilent 1500 B analyzer equipped with a four-point probe was used to measure the sheet resistance of porous NiSi substrates. An electrolyte conductivity meter (Salinity meter, CON 500, Clean Instruments) was used to measure changes in conductivity in the solutions during permeability test. The mechanical property of the porous NiSi membrane was examined using a three-point flexural test.Setup for testing permeability of membrane
The platform for the permeability test was made by two Pyrex glass chambers with top-down sets separated by a channel area of 1 cm2. The HfO2/porous NiSi membranes were stacked by two O-rings and then mounted on the test platform during the desalination test. The desalination membranes divided this platform into two separate chambers. During the experimental setup, the top chamber was loaded with 0.5 M NaCl solutions, while the bottom chamber was filled with pure water; a conductivity meter was placed in a fixed position in the bottom chamber for the in situ monitoring of conductivity changes.Results and discussion
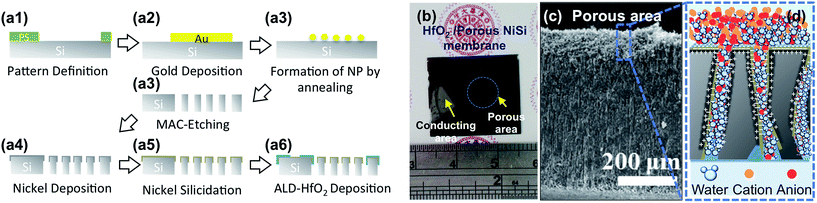
Fig. 1(a) shows a concise fabrication schematic of ALD-HfO2/porous NiSi hierarchical structures and their application in desalination (see Experimental section). A thin gold film was selectively deposited on a Si substrate by an e-gun evaporator (Fig. 1(a1 and a2)), followed by post-annealing treatment at temperatures of 700–1000 °C to obtain Au NP arrays (Fig. 1(a3)). The corresponding SEM images at various Au thicknesses and annealing temperatures are shown in Fig. S1.† It indicates that both the inter-spacing between the NPs and the size of NPs are larger with thicker deposited films. In fact, spacing between the NPs should be sufficiently large to prevent the immoderate formation of high-porosity structures induced by the time-consuming MACE process. Obviously, the annealing temperature of 1000 °C on 20 nm-thick Au film can match our requests, which can be applied as catalysts for time-consuming MACE process.10 Subsequently, the samples were placed in a mixed solution containing HF (49 wt%, ECHO Chemical Co.), H2O2 (30 wt%, Showa Chemical Industry Co.) and deionized water to obtain porous Si structures. Both the sides of the porous Si structure were then mechanically polished to produce smoother surfaces for subsequent manufacturing processes. Nickel silicide was formed on the surface-polished porous Si substrate by the deposition of a thin film of Ni with post-thermal treatment (Fig. 1(a4–a6)). To avoid direct contact of the conducting layer with the solution in a practical application, a uniform dielectric layer, HfO2, was deposited on the surface of the porous NiSi substrate using the ALD technique. Technically, our manufacturing is compatible with the semiconductor industry. The photograph of the as-formed HfO2/porous NiSi membrane is shown in Fig. 1(b). Metal-like reflection on the conducting areas and a dark-colored appearance on the porous areas due to the deposition of the ALD-HfO2 layer can be observed. In addition, the porous structures were etched using the MACE methods for 24 h to obtain better porosity through the wafers, as shown in Fig. 1(c).12 Fig. 1(d) illustrates that the selective ion diffusion was achieved by concept of EDLs overlapping. The applied positive bias more easily induces the accumulation of negatively charged ions (anions) on the HfO2 surface to further electrically occupy the sidewalls of channels via surface polarization as EDLs.13 Therefore, in this case, the diffusion of cations will be restrained by the application of positive bias through the bottom chamber. In the case when negative bias is applied, anions will be excluded in the channels of the HfO2/porous NiSi membranes, allowing the diffusion of cations through the bottom chamber. To optimize the formation of porous Si substrates by the MACE condition, the surface morphologies of these porous Si structures were examined using various compositions of etching solution.Herein, we set a dimensionless parameter ρ, which represents the molar ratio of HF divided by HF + H2O2 with an etching time of 20 min. The corresponding tilted-view SEM images of these porous structures etched using different ρ from 0.55 to 0.92 are shown in Fig. 2. Obviously, the as-formed pores appear as straight-like channels with the lower ρ (Fig. 2a and b) and turn porous-like with higher ρ (Fig. 2c and d). It is believed that higher HF content (higher ρ) might lead to porous-like morphology because the by-product, SiF62−, can be easily formed, resulting in random etching behavior, which is consistent with the report from the literature.14 Consequently, the highest pore density and smoothest etched surfaces can be achieved using the etching solution with ρ at 0.92. However, we find that the etched porous structure with ρ at 0.92 was too brittle to proceed for following manufacturing. Accordingly, the etching solution with ρ at 0.86, which penetrated the entire wafer thickness, was selected as the best parameter in our current study. To further observe the etching behaviors with ρ at 0.86, an etching time from 20 min to 24 h was conducted. Obviously, pore size increased with the etching time, while the etched surface became rougher with longer etching time. The rougher surface at longer etching time may be attributed to the direct etching of the porous Si surface by etchants or assisted etching through metal NPs on the surface due to random seeking direction.15,16 To summarize our observation results on the formation of porous Si by the MACE process, the average diameter and length of the pore channels were obtained and plotted as a function of the etching time, as shown in Fig. 3(a1–a4), (b), (c1–c4) and (d). Obviously, the length of the pore channels increased with the longer etching time, while saturation occurred when the etching time was over 5 h (Fig. 3(c)). Note that the diameter of pore channels is apparently found to be dramatically increased once the MACE process was performed for more than 3 h (Fig. 3(d)). However, the observed saturation of pore widening leads to the diameter of the etched pore as large as ∼1 μm after etching for 5 h or longer, which is consistent with previous reports.11,17
 | ||
| Fig. 2 Tilted-view SEM images of porous Si structures etched using solutions of various ρ at (a) 0.55, (b) 0.71, (c) 0.86, and (d) 0.92. | ||
The formation of the porous Si structure will further degrade its conductivity, making the overall porous Si substrate more resistive. To tackle this problem while maintaining an unchanged porous configuration, the formation of a low resistive porous silicide is an alternative. Among all the types of silicide, nickel silicide is the best choice because of lowest resistivity.18 In previous studies, the stoichiometric variation of nickel silicide led to different electrical properties.18–20 Theoretically, the sheet resistance of the NiSi phase can reach as low as 1–10 Ω □−1, which matches our requirement; thus, a conducting layer with excellent electrical properties is obtained. To achieve such a low value of sheet resistance, a 200 nm-thick sputtering Ni film was deposited on the as-prepared porous Si template with additional post-annealing to obtain a conductive porous NiSi configuration. Note that the annealing temperature profile is included in Fig. S2,† where the annealing temperatures were applied according to the similar previous studies.20 Fig. 4(a) illustrates the grazing incidence X-ray diffraction (GIXRD) of the as-deposited Ni layer on the porous Si configuration before and after the post-annealing process. Obviously, crystalline Ni and Si phases can be confirmed from GIXRD results; they match JCPDS 04-0850 and 80-0018, whereas the formation of the NiSi phase can be confirmed by JCPDS 38-0844 after the post-annealing treatment. In addition, no obvious morphological change can be found before and after the silicidation process, as shown in Fig. 4(b) and (c). Therefore, the sheet resistance of the porous NiSi configuration was calculated to be ∼2.3 Ω □−1.
 | ||
| Fig. 4 (a) X-ray diffraction patterns of porous NiSi structures before and after post-thermal annealing. The corresponding SEM images (b) before and (c) after post-thermal annealing. | ||
Furthermore, a device was used to test the electrical charge-induced selective ions permeation in HfO2/porous NiSi membranes, in which the membrane was inserted as a filter stacked by two solution chambers with a top-down set, as shown in Fig. 5(a) (see Experimental section in the ESI†). Furthermore, electrolyte conductivity changes in the bottom chamber were monitored to examine deionization ability owing to the electrical charge-induced selective ionic penetration in the HfO2/porous NiSi membrane with different applied biases by filling the top and bottom solution chambers with 0.5 M NaCl solution and pure water (Fig. 5(a)), respectively. The relation between the salt concentration and the measured conductivity were examined as the reference with a slope of 97.8 μS mM−1 cm−1 (Fig. S3†). By applying different external biases of 1 V and 2 V, the deionization ability of the HfO2/porous NiSi membrane can be examined, as shown in Fig. 5(b), using which the deionization ability of the HfO2/porous NiSi membrane without the applied bias was plotted, as well. Interestingly, the diffusion of ions from the top to the bottom chambers resulted in an increase of ion concentration in the bottom solution chamber with an increase in time. However, the tendency of ionic concentration decreased when the applied bias was increased from 1 V to 2 V. As a result, after a diffusion duration of 6 h, a decrease in ionic concentration from 9.7 mM to 8.6 mM was observed with an applied bias of 1 V, whereas once the applied bias was further increased to 2 V, a continuous decrease from 8.6 mM to 7.4 mM was observed. Therefore, a maximum reduction in ionic concentration of 21% can be achieved by applying a bias of 2 V after a diffusion duration of 6 h.
In addition, the HfO2/porous NiSi membrane can be continuously operated without the loss of its deionization ability for at least 18 h. EDL overlapping effect facilitates the movement of electrical charge-induced selective ions permeation contributed to the decrease of ionic concentration in bottom solution chamber. Note that the saline water containing anions and cations can pass through the channels of the HfO2/porous NiSi membrane without any applied bias, indicating that no desalination occurs. However, the same electrical charge-induced selective ions in the channels of the HfO2/porous NiSi membrane channels when induced by either positive or negative biases may create differences in diffusivity (diffusion coefficient) between cations and anions, resulting in different desalination ability. For further exploration, a diffusion test relating to membrane permeability by the application of positive and negative biases of 1 V and −1 V was carried out, and is shown in Fig. 5(b). Obviously, the quantity of the reduced ionic concentration triggered by the negative bias is more apparent than that by the positive. The variation in diffusion test results can be explained through differences in ionic diffusivity. For the applied positive bias, the diffusion of cations will be restrained by the induced positive electrical field in HfO2/porous NiSi membrane nanochannels, allowing for the diffusion of anions in this case. However, in the case of applied negative bias, anions will be excluded in the nanochannels of the HfO2/porous NiSi membranes, allowing the diffusion of cations through the bottom chamber. By considering our case as an example, the diffusion coefficients of sodium (Na+) and chloride ions (Cl−) in an electrolyte solution were evaluated to be 13.3 and 20.3 × 10−6 cm2 s−1 at room temperature, respectively.21 Therefore, the slower diffusivity of cations (Na+ ions) compared to that of anions (Cl− ions), reasonably explains the lower ionic concentration in the bottom chamber once the negative bias was applied.
To consider the practical application of the desalination process, membranes with good mechanical properties against fluidic resistance will be imperative. Therefore, the mechanical properties of the HfO2/porous NiSi membrane were examined using a three-point flexural test using the HfO2/Al/AAO membrane as the reference for comparison. Fig. 6(a) indicates the load-extension curves of HfO2/porous NiSi and HfO2/Al/AAO membranes, respectively, and the magnified load-extension curve of the HfO2/Al/AAO membranes is shown in Fig. 6(b). The corresponding schematic of how we performed three-point flexural test is shown in the inset of Fig. 6(a). The abrupt fracture from load-extension text distinctly indicates the brittle behavior of the two membranes.22,23 For a specimen with a rectangular beam, the flexural strength (in MPa) can be defined as  where F, w, t and L represent the maximum load F applied at the middle of beam before fracture, the width and thickness of the beam, and the distance between the two span supports, respectively.24 For the HfO2/Al/AAO membrane, flexural strength was examined and calculated to be 60.78 MPa at the lower maximum load of 0.15 N with a maximum extension of ∼0.57 mm, as listed in Table 1. Conversely, an enhanced flexural strength of 124.04 MPa (Table 1) and mechanical strength reaching to 13.2 N with a maximum extension of ∼0.04 mm can be analyzed before fracture for the HfO2/porous NiSi membrane (Fig. 6(b)). The findings suggest that the HfO2/porous NiSi membrane with enhanced mechanical property can be considered as a processable material in desalination.
where F, w, t and L represent the maximum load F applied at the middle of beam before fracture, the width and thickness of the beam, and the distance between the two span supports, respectively.24 For the HfO2/Al/AAO membrane, flexural strength was examined and calculated to be 60.78 MPa at the lower maximum load of 0.15 N with a maximum extension of ∼0.57 mm, as listed in Table 1. Conversely, an enhanced flexural strength of 124.04 MPa (Table 1) and mechanical strength reaching to 13.2 N with a maximum extension of ∼0.04 mm can be analyzed before fracture for the HfO2/porous NiSi membrane (Fig. 6(b)). The findings suggest that the HfO2/porous NiSi membrane with enhanced mechanical property can be considered as a processable material in desalination.
| No. | Max. load (N) | Length (mm) | Width (mm) | Thickness (mm) | Flexural strength (MPa) |
|---|---|---|---|---|---|
| HfO2/porous NiSi | 13.231 | 10 | 10 | 0.4 | 124.036 |
| HfO2/AL/AAO | 0.146 | 10 | 10 | 0.06 | 60.783 |
Although phenomenon regarding electrical charge-induced selective ion permeation are demonstrated in HfO2/porous NiSi hierarchical structures, further investigation to improve the understanding of nanoscale transport phenomena and the development of nanostructured material manufacturing techniques is needed for the realization of highly efficient, affordable and sustainable seawater desalination to meet the ongoing demand for clean water sources. Furthermore, to achieve one-time saline water desalination, the multi-assembly of HfO2/porous NiSi membranes with biases of different polarities is a key issue for improvements in desalination ability, which can simultaneously remove anions and cations. More importantly, our approach can be applied not only in water desalination but also in ion-separation technique.
Conclusion
We have successfully fabricated filterable porous NiSi templates through metal-assisted chemical etching followed by the deposition of Ni layer and silicidation process. A demonstration of the desalination of saline water by manipulating electrical charge-induced selective ion penetration in HfO2/porous NiSi hierarchical structures was achieved through diffusion testing to better understand the permeability of the porous NiSi membrane. Ionic concentration was reduced by 21% with a maximum applied bias of 2 V, which indicated that the desalination abilities of membranes were different when biases with different polarities were applied owing to the diffusivity difference between cations and anions. These HfO2/porous NiSi membranes can be continuously operated without the loss of its deionization ability for at least 18 h. Its flexural strength was examined and calculated to be 124.04 MPa, which suggests that it is mechanically stronger than the AAO membrane we used in our previous study. Our results show that the HfO2/porous NiSi membrane has potential as a processable material for application in desalination.Acknowledgements
C. C. L. and C. H. Lu thank Mr Hao-Jen Fang and Mr Teng-Yu Su for their assistance in three-point flexural test and four-point probe measurements. The research is supported by the Ministry of Science and Technology through Grant nos 101-2112-M-007-015-MY3, 101-2218-E-007-009-MY3, and 103-2633-M-007-001, and the National Tsing Hua University through Grant no. 104N2022E1. Y. L. Chueh greatly appreciates the use of facility at CNMM, National Tsing Hua University through Grant no. 104N2744E1.Notes and references
- J. Bartram, Nature, 2008, 452, 283–284 CrossRef CAS PubMed.
- R. Semiat, Environ. Sci. Technol., 2008, 42, 8193–8201 CrossRef CAS.
- N. Ghaffour, T. M. Missimer and G. L. Amy, Desalination, 2013, 309, 197–207 CrossRef CAS PubMed.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- Global Water Intelligence (GWI/IDA Desal Data), Market profile and desalination market, 2009–2012 yearbooks and GWI website, http://www.desaldata.com/.
- G. M. Geise, H. B. Park, A. C. Sagle, B. D. Freeman and J. E. McGrath, J. Membr. Sci., 2011, 369, 130–138 CrossRef CAS PubMed.
- S. J. Kim, S. H. Ko, K. H. Kang and J. Han, Nat. Nanotechnol., 2010, 5, 297–301 CrossRef CAS PubMed.
- P. Kim, S. J. Kim, J. Han and K. Y. Suh, Nano Lett., 2010, 10, 16–23 CrossRef CAS PubMed.
- T. Humplik, J. Lee, S. C. O'Hern, B. A. Fellman, M. A. Baig, S. F. Hassan and E. N. Wang, Nanotechnology, 2011, 22, 292001 CrossRef CAS PubMed.
- C. C. Lai, C. J. Chang, Y. S. Huang, W. C. Chang, F. G. Tseng and Y. L. Chueh, Nano Energy, 2015, 12, 394–400 CrossRef CAS PubMed.
- Z. Huang, N. Geyer, P. Werner, J. De Boor and U. Gösele, Adv. Mater., 2011, 23, 285–308 CrossRef CAS PubMed.
- S. Cruz, A. Hönig-d'Orville and J. Muller, J. Electrochem. Soc., 2005, 152, C418–C424 CrossRef CAS PubMed.
- F. Baldessari, J. Colloid Interface Sci., 2008, 325, 526–538 CrossRef CAS PubMed.
- X. Li, Curr. Opin. Solid State Mater. Sci., 2012, 16, 71–81 CrossRef CAS PubMed.
- F. Guder, Y. Yang, U. M. Kucukbayrak and M. Zacharias, ACS Nano, 2013, 7, 1583–1590 CrossRef PubMed.
- S. Y. Li, W. H. Ma, Y. Zhou, X. H. Chen, Y. Y. Xiao, M. Y. Ma, F. Wei and X. Yang, J. Solid State Chem., 2014, 213, 242–249 CrossRef CAS PubMed.
- I. Leontis, A. Othonos and A. G. Nassiopoulou, Nanoscale Res. Lett., 2013, 8, 383–390 CrossRef PubMed.
- O. Nakatsuka, K. Okubo, A. Sakai, M. Ogawa, Y. Yasuda and S. Zaima, Microelectron. Eng., 2005, 82, 479–484 CrossRef CAS PubMed.
- Y. C. Lin, Y. Chen, D. Xu and Y. Huang, Nano Lett., 2010, 10, 4721–4726 CrossRef CAS PubMed.
- R. Tomita, H. Kimura, M. Yasuda, K. Maeda, S. Ueno, T. Tonegawa, T. Fujimoto, M. Moritoki and H. Iwai, Microelectron. Reliab., 2013, 53, 665–669 CrossRef CAS PubMed.
- Y. H. Li and S. Gregory, Geochim. Cosmochim. Acta, 1974, 38, 703–714 CrossRef CAS.
- S. H. Ko, D. W. Lee, S. E. Jee, H. C. Park, K. H. Lee and W. Hwang, Glass Phys. Chem., 2005, 31, 356–363 CrossRef CAS PubMed.
- K. Y. Ng, PhD Thesis, The University of Hong Kong, Pokfulam, Hong Kong, 2009.
- J. B. Wachtman, Mechanical properties of ceramics, Wiley, New York, 1996, vol. 76 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM images of Au NP arrays obtained under optimally annealed conditions; heating profile for the formation of the NiSi conductive phase; calibration of electrolyte conductivity versus salt concentration; flexural strength of porous NiSi and AAO membrane by three-point flexural testing. See DOI: 10.1039/c5ra03278d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |