N-Trinitroethyl-substituted azoxyfurazan: high detonation performance energetic materials†
Abstract
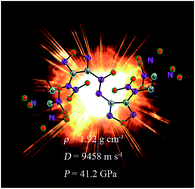
Azoxyfurazan derivatives based on the trinitroethyl functionality were synthesized. These energetic N-trinitroethyl-substituted azoxyfurazans were fully characterized by using 1H and 13C NMR spectroscopy, IR, elemental analysis, differential scanning calorimetry (DSC), thermogravimetric analysis (TG) as well as single crystal X-ray diffraction, and, in the case of N-trinitroethylamino azoxyfurazan 4, with 15N NMR spectroscopy. Furthermore, compound 4 and nitramine 5 have been tested for their responses to impact, friction, and electrostatic discharge. The detonation pressures and velocities of the azoxyfurazan derivatives were calculated, ranging from 35.8 GPa to 41.2 GPa and 8861 m s−1 to 9458 m s−1, respectively. Additionally, compound 5 having an oxygen balance of near zero (+2.5%), exhibits a favorable measured density (1.92 g cm−3) and excellent detonation property (ΔfHm, 962.1 kJ mol−1; P, 41.2 GPa; D, 9458 m s−1). Thus, these compounds could be potential high detonation performance energetic materials.

 Please wait while we load your content...
Please wait while we load your content...