Novel efficient fluorophores synthesized from citric acid†
Wiktor Kasprzyk*a,
Szczepan Bednarza,
Paweł Żmudzkib,
Mateusz Galicaa and
Dariusz Bogdała
aDepartment of Biotechnology and Physical Chemistry, Faculty of Chemical Engineering and Technology, Cracow University of Technology, 24 Warszawska St., 32-048 Krakow, Poland. E-mail: jumper.wk@gmail.com
bDepartment of Medicinal Chemistry, Jagiellonian University Medical College, 9 Medyczna St., 30-688 Kraków, Poland
First published on 9th April 2015
Abstract
A series of novel fluorescent compounds was isolated from condensation mixtures of citric acid and specific β-amines. Their chemical structure was determined using 1H, 13C and HSQC experiments, elemental analysis data, high resolution and fragmentation spectra ESI-MS analyses. These compounds consisted of five-membered ring fused 2-pyridones. The fluorophores exhibited quantum yields as high as 79%.
Recently, fluorescent materials synthesized from renewable resources have gained great attention mainly because of low costs of their synthesis and high quantum efficiency. Citric acid (CA) has become one of the most frequently used bioproducts for the synthesis of luminescent materials.1 Trailblazing papers in the field of citric acid based fluorescent materials (CAFM) were published in 19th century by W. J. Sell and T. H. Easterfield. They noticed that CA is able to form fluorescent citrazinic acid as a result of condensation reaction with ammonia.2 Later on CA was reacted with specific aliphatic and aromatic β-amino thiols to produce highly fluorescent five membered ring fused 2-pyridones.3,4 Recently, many authors have reported synthesis of fluorescent carbon and graphene dots prepared through pyrolysis of citric acid (CCD). These materials possess a number of advantages such as biocompatibility, straightforward synthesis and excitation dependent emission spectra but also have one important drawback i.e. relatively low quantum yield (QY) ∼ 10%.5–7 Therefore, a number of researchers utilized various amine-based surface passivation agents in order to overcome this problem.8–28 As a result it was possible to increase the QY of these materials to ∼14% by doping with urea and up to ∼90% by doping with 1,2-ethylenediamine.29,30 These materials target many possible applications e.g. pH sensors, biomarkers, fluorescent inks, solar cells, fluorescent probes for detection of metals and biomolecules.8–30 Despite of many papers that have been recently published in this area, the origin of fluorescent properties of these materials has not been fully described.16,19 The formation of carbon dots was well established in the literature and this is a good explanation for excitation dependent fluorescent properties of CCD.7 On the other hand, the QY build-up of CCD synthesized in the presence of N, S and O sources was considered as a result of N-doping, formation of amide bonds on the surface of CCD,8 enhancement of N-surface states by co-doping with sulfur,16 or formation of organic fluorophores.19 Nevertheless, the origin of so high QY in carbon dots prepared from CA and some specific amines is still unknown. The great potential of CA to form fluorescent molecules in reaction with amines was used to fabricate a set of biodegradable photoluminescent polymers (BPLP).31 Moreover, BPLPs demonstrate in vitro cytocompatibility, minimal chronic inflammatory responses in vivo, controlled degradability, good photostability, excellent processability for micro/nano fabrication and desired mechanical properties.31–33 Furthermore, these polymers have recently been employed in the synthesis of Dual-Imaging Enabled Cancer-Targeting Nanoparticles.34 Lately, the possible origin of their luminescent properties has been revealed by isolation and identification of luminescent agent from the hydrolysate of BPLP. Detailed analyses confirmed the chemical structure as derivative of 2-pyridone i.e. 5-oxo-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyridine-3,7-dicarboxylic acid.35 Similar 2-pyridone based structures have been suspected as an origin of luminescent properties of fluorescent mesoporous silica.36
Herein, we describe the structure and spectroscopic properties of the fluorescent compounds that are formed during heating of CA in the presence of various α,β-bifunctional amines. In this research, we use amines which are known to form optically efficient fluorescent materials in reaction with CA as well as we propose some new structure motifs which could be readily introduced in their synthesis. We also suggest general chemical structure of β-substituted amines which can form highly fluorescent moieties in reaction with CA.
Literature studies revealed that the highest QY of CA based materials was achieved using α,β-diamines, β-amino thiols and β-amino alcohols as precursors.8–28,31 On the other hand, various carboxylic acids were examined as carboxyl sources instead of CA in the synthesis of fluorescent materials. However, none of the studied compounds, in reaction with 1,2-ethylenediamine or L-cysteine, produced a moiety exhibiting QY higher than those fabricated from CA.14,31 Therefore, we hypothesized that CA is able to react with specific α,β-diamines, β-amino thiols and β-amino alcohols (Fig. 1) to produce fluorescent organic dyes. In order to examine this thesis, we reacted CA with five different bifunctional amines separately (1a–5a) at 180 °C for 1 h. Afterwards, fluorescent products (1b–5b) were isolated using preparative HPLC. Then their chemical structure was determined using 1H, 13C, HSQC NMR experiments (Fig. S1A–E†), elemental analysis (Table S1†), ESI-MS/MS (Fig. S2A–J†) and HR-ESI-MS (Table S1†). We found that fluorescent fractions consist of expected ring fused 2-pyridones, similar to those reported earlier.3,4,35 We were able to fabricate and isolate several pure fluorescent compounds i.e. 5-oxo-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyridine-7-carboxylic acid (1b), 5-oxo-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyridine-3,7-dicarboxylic acid (2b), 1-oxo-1H-pyrido[2,1-b][1,3]benzoxazole-3-carboxylic acid (3b), 1-oxo-1H-pyrido[2,1-b][1,3]benzothiazole-3-carboxylic acid (4b), and 1-oxo-1,5-dihydropyrido[1,2-a]benzimidazole-3-carboxylic acid (5b) (Fig. 1) trough condensation reaction of CA and cysteamine (1a), L-cysteine (2a), o-aminophenol (3a), o-aminothiophenol (4a) and o-phenylenediamine (5a), respectively. The choice of the amine substrates was based on the feasibility of isolation of pure fluorescent products from reaction mixtures.
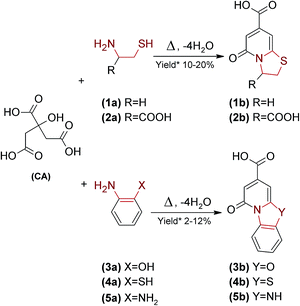 | ||
| Fig. 1 Formation of fluorescent 2-pyridones from CA and selected β-substituted amines. (*) Yield estimated by HPLC. | ||
According to Olthoff et al. the mechanism of the formation of compounds very similar to those fabricated herein consists of formulation of an amide bond between CA and amine and few intramolecular condensation steps i.e. formation of 2-pyridone ring, dehydration, forming of five-membered ring fused 2-pyridone by intramolecular condensation of thiol group with pyridone oxygen.3 We consider the latter step also feasible in case of formation of fluorescent compounds from CA and other nucleophiles such as α,β-diamines or β-amino alcohols. Acid catalysed condensation reactions involving electrophilic attack of an acid on carbonyl oxygen followed by nucleophilic attack of alcohols, thiols and amines on carbonyl carbon of ketones were frequently reported.37
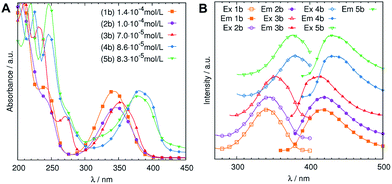
We examined UV-vis absorption, fluorescence excitation and emission spectra and determined quantum yields and fluorescence lifetimes of water solutions of synthesized compounds (1b–5b). We found that they all show strong absorption bands around 260 nm and 350 nm (Fig. 2A). We consider the former bands are attributed to the π → π* transition of sp2 carbons of aromatic and heterocyclic rings, while the latter band is originated from the n → π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonding. Most of the above compounds show concentration quenching mechanism of fluorescence with the most efficient fluorescence emission in concentration as low as ∼3.5 × 10−4 mol L−1 (Fig. S3A–E†). This is a crucial fact bearing in mind that many authors conduct optimization of CAFM synthesis conditions basing on the fluorescence intensity of achieved products. The water solutions show very bright violet-blue or blue luminescence with absolute QY above 60% with the highest achieved for (3b) 79% (Table S2†). These QY's values are comparable to those reported for other fluorescent organic dyes such as some rhodamines and coumarines (40–90%).38 Solutions of compounds 1b–5b show excitation independent fluorescence with maximum excitation wavelength around 350 nm and maximum emission wavelength 418–450 nm depending on the examined compound (Fig. 2B). Moreover, emission spectra display broad, non-symmetrical shape with the long-wavelength tail common to most of organic fluorophores.38 Fluorescence decay curves of 1b–5b indicate typical exponential fluorescence decay behaviour for most of the compounds (Fig. S4A–E†). The fluorescence lifetimes were in range 0.2–8.24 × 10−9 s (Table S2†) which are common values for fluorescent organic dyes (<10 ns).38 Water solutions of 1b–5b exhibit stokes shifts as high as 5390 cm−1 (Table S2†). We conclude that the source of fluorescence is reasonably related to π → π* and, to a lesser extent, n → π* electron transitions.
O bonding. Most of the above compounds show concentration quenching mechanism of fluorescence with the most efficient fluorescence emission in concentration as low as ∼3.5 × 10−4 mol L−1 (Fig. S3A–E†). This is a crucial fact bearing in mind that many authors conduct optimization of CAFM synthesis conditions basing on the fluorescence intensity of achieved products. The water solutions show very bright violet-blue or blue luminescence with absolute QY above 60% with the highest achieved for (3b) 79% (Table S2†). These QY's values are comparable to those reported for other fluorescent organic dyes such as some rhodamines and coumarines (40–90%).38 Solutions of compounds 1b–5b show excitation independent fluorescence with maximum excitation wavelength around 350 nm and maximum emission wavelength 418–450 nm depending on the examined compound (Fig. 2B). Moreover, emission spectra display broad, non-symmetrical shape with the long-wavelength tail common to most of organic fluorophores.38 Fluorescence decay curves of 1b–5b indicate typical exponential fluorescence decay behaviour for most of the compounds (Fig. S4A–E†). The fluorescence lifetimes were in range 0.2–8.24 × 10−9 s (Table S2†) which are common values for fluorescent organic dyes (<10 ns).38 Water solutions of 1b–5b exhibit stokes shifts as high as 5390 cm−1 (Table S2†). We conclude that the source of fluorescence is reasonably related to π → π* and, to a lesser extent, n → π* electron transitions.
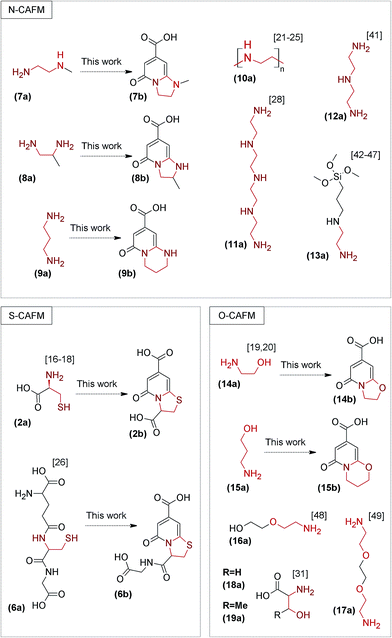
All foregoing considerations concerned the synthesis, chemical structure and spectroscopic features of fluorophores derived from reaction of CA with α,β-diamines, β-amino thiols or β-amino alcohols. As we noticed at the beginning of this paper literature studies revealed that citric acid based materials with the highest QY were fabricated in reaction with analogous β-amine precursors. Thus, the preceding studies focus on the detection of analogous fluorophores during reaction of CA with other α,β-bifunctional amines. Because of the high diversity of β-amine precursors reported as dopants for the synthesis of CAFM (2a, 6a–17a) we decided to divide these into three main groups. We did it according to the nucleophilic group at the β-position in bifunctional amine i.e. thiol (S-CAFM), amine (N-CAFM) and alcohol (O-CAFM) (Fig. 3). Then, we chose one or two representative β-amine precursors from each group to react with CA and examine the presence of ring fused 2-pyridone moieties in the reaction mixture using mass spectrometry methods (ESI-MS/MS and HR-ESI-MS). These methods allow separation of complex reaction mixtures and detection of desired compounds even at very low concentrations. Moreover, the combination of HR-LCMS data with fragmentation spectra of molecular ions have been frequently reported as a sufficient method for structure elucidation of peptides, biomolecules as well as small molecules and metabolites.39,40
The S-CAFM group has been represented in the literature up to now only by CAFM synthesized in the presence of (2a), (4a) and glutathione (6a).16–18,26,31 Herein we confirmed that (2b) can be easily fabricated through condensation reaction of CA and (2a). In case of S-CAFM synthesized from CA and (6a) we found analogous dye as one of the sources of their fluorescence properties (6b). The mixture of CA and (6a) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was heated for 1 h at 180 °C. Then it was analysed using ESI-MS/MS (Fig. S5A†) and HR-ESI-MS (Table S3†). We suppose that both acidity of CA and elevated temperature of the reaction impart the hydrolysis of (6a). Then cysteine–glycine dipeptide reacts with CA in the same manner as (2a) during the synthesis of (2b). We found appropriate molecular ion with molecular formula C11H10N2O6S. Furthermore, fragmentation pattern of this molecular ion suggests the chemical structure of 3-[(carboxymethyl)carbamoyl]-5-oxo-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyridine-7-carboxylic acid (6b) (Fig. S5B†). All these findings indicate the presence of ring fused 2-pyridones during the synthesis of both (2a) and (6a) doped CAFM.
1) was heated for 1 h at 180 °C. Then it was analysed using ESI-MS/MS (Fig. S5A†) and HR-ESI-MS (Table S3†). We suppose that both acidity of CA and elevated temperature of the reaction impart the hydrolysis of (6a). Then cysteine–glycine dipeptide reacts with CA in the same manner as (2a) during the synthesis of (2b). We found appropriate molecular ion with molecular formula C11H10N2O6S. Furthermore, fragmentation pattern of this molecular ion suggests the chemical structure of 3-[(carboxymethyl)carbamoyl]-5-oxo-2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyridine-7-carboxylic acid (6b) (Fig. S5B†). All these findings indicate the presence of ring fused 2-pyridones during the synthesis of both (2a) and (6a) doped CAFM.
N-CAFM group is composed of α,β-diamine derivatives doped materials. Taking into account high reactivities and high molecular weights of some α,β-diamine substrates,8–16,21–25 we decided to use low molecular weight model compounds in order to get better understanding of the origin of fluorescence properties of N-CAFM group. Therefore, we chose N-methyloethylenediamine (7a) as a model because of similarities in the chemical structure to poly(ethyleneimine) (10a), tetraethylenepentamine (11a), diethylenetriamine (12a) and [3-(2-aminoethylamino)propyl]trimethoxysilane (13a). All above compounds are known to form fluorescent CCD in reaction with CA.21–25,28,41–47 Therefore, we reacted (7a) with CA and then we analysed a sample of the reaction mixture using HR-ESI-MS. This analysis showed the presence of a ion with m/z = 195.0769. The molecular formula of this ion was C9H10N2O3 which correlates to chemical composition of proposed fluorescent dye i.e. 1-methyl-5-oxo-1,2,3,5-tetrahydroimidazo[1,2-a]pyridine-7-carboxylic acid (7b). Moreover, the ESI-MS/MS fragmentation spectra (Fig. S6A†) of this ion also matches the chemical structure of proposed compound (Fig. S6B†). In case of 1,2-ethylenediamine doped N-CAFM8–16 we used its derivative namely 1,2-diaminopropane (8a) as a model compound. Similarly, we found appropriate molecular ion in HR-ESI-MS spectrum with molecular formula C9H10N2O3. Moreover, the ESI-MS/MS fragmentation spectra (Fig. S7A†) for this 2-methyl-5-oxo-1,2,3,5-tetrahydroimidazo[1,2-a]pyridine-7-carboxylic acid (8b) suggest proposed chemical formula (Fig. S7B†).
The third group consist of β-amino alcohol derivatives doped CCD and BPLPs (O-CAFM). We have found couple of reports describing synthesis of CAFM passivated by ethanolamine (14a),19,20 L-serine (18a) and L-threonine (19a).31 Furthermore, some authors reported 2-(2-aminoethoxy)-ethanol (16a) and 2,2′-(ethylenedioxy)bis(ethylamine) (17a) as CCD surface passivation agents.48,49 Taking into account possibility of ether hydrolysis at high temperatures and acidic pH,50 we suppose that above compounds are able to produce small amounts of (14a) in reaction conditions applied for CAFM synthesis. Therefore, we found reaction of CA with (14a) as an appropriate example to explain fluorescent properties of O-CAFM group. Thus, the HR-ESI-MS analysis (Table S1†) revealed the presence of molecular ion with molecular mass and composition of 5-oxo-2,3-dihydro-5H-[1,3]oxazolo[3,2-a]pyridine-7-carboxylic acid (14b). ESI-MS/MS fragmentation spectra of this molecular ion also indicates proposed chemical structure (Fig. S8A and S8B†). Formation of this kind of organic dyes seems to be a good complement to the thesis made by Krysmann et al.19 The authors supposed that during the reaction of CA with (14a) at temperatures < 200 °C mostly some organic, strongly fluorescent chromophores are formed. As the pyrolysis proceeds to higher temperatures a carbogenic core is formulated at the expense of these fluorophores. Thus, Krysmann et al. provided formation mechanism of CCD with dual photoluminescence emission i.e. high QY, excitation independent caused by organic fluorophores and low QY, excitation dependent as a result of formation of carbogenic cores.19 However, the chemical structure of these fluorophores has not been proposed until now.
All above examinations revealed the formation of specific five-membered ring fused 2-pyridone organic dyes as a result of condensation of CA with α,β-diamine, β-amino thiol or β-amino alcohol derivatives (1b–8b, 10b–14b, 16b, 17b). Furthermore, we examined also α,γ-bifunctional amines i.e. 1,3-diaminopropane (9a) and 3-amino-1-propanol (15a) in terms of possible formation of six-membered ring fused 2-pyridone structures after heating with CA (Fig. S9†). As a result we produced fluorescent mixtures which were examined using mass spectrometry analyses. We found appropriate ions in HR-ESI-MS spectra (Table S1†) with molecular composition C9H10N2O3 and C9H10NO4 for dyes synthesized from (9a) and (15a), respectively. Moreover, the fragmentation spectra (Fig. S10A and S11A†) of these ions indicate the presence of expected six-membered ring fused 2-pyridones (9b) and (15b) (Fig. S10B and S11B†). According to our best knowledge the CAFM produced from these kind of amines have not been reported up to now.
Conclusions
In conclusion, we found that CA is able to react with specific α,β- and α,γ-bifunctional amines to produce five and six-membered ring fused 2-pyridone based fluorescent organic dyes. These compounds exhibit QY as high as 79%. We have proven that water solutions of these compounds in concentration as low as 3.5 × 10−4 mol L−1 are able to emit strong luminescence. We proposed several amine reagents which can readily be used in the synthesis of novel very efficient CA based fluorescent materials. Bearing in mind constantly growing interest in CAFM, the significance of our development seems to be indisputable. Studies on the influence of possible presence of similar compounds in the chemical structure of amine doped CCD on their quantum efficiency are underway and will be the subject of future publications.Acknowledgements
The authors gratefully acknowledge the support for this work from the National Centre of Science of Poland (grant number: 2011/01/N/ST5/05591). The research was also supported by the European Union through the European Social Fund (contract no. UDA-POKL.04.01.01-00-029/10-00) and (contract no. UDA-POKL.04.01.02-00-217/11-00).Notes and references
- K. Hola, Y. Zhang, Y. Wang, E. P. Giannelis, R. Zboril and A. L. Rogach, Nano Today, 2014, 9, 590–603 CrossRef CAS.
- W. J. Sell and T. H. Easterfield, J. Chem. Soc., Trans., 1893, 63, 1035–1051 RSC.
- U. Olthoff, R. Hüttenrauch and K. Matthey, Tetrahedron, 1971, 27, 3447–3455 CrossRef CAS.
- M. Hori, T. Kometani, H. Ueno and H. Morimoto, Biochem. Med., 1974, 11(1), 49–59 CrossRef CAS PubMed.
- A. Zhu, C. Ding and Y. Tian, Sci. Rep., 2013, 3, 2933 Search PubMed.
- Z. Wu, W. Li, J. Chen and C. Yu, Talanta, 2014, 119, 538–543 CrossRef CAS PubMed.
- Y. Dong, J. Shao, C. Chen, H. Li, R. Wang, Y. Chi, X. Lin and G. Chen, Carbon, 2012, 50, 4738–4743 CrossRef CAS.
- X. Y. Zhai, P. Zhang, C. J. Liu, T. Bai, W. C. Li, L. M. Dai and W. G. Liu, Chem. Commun., 2012, 48, 7955–7957 RSC.
- F. Yan, Y. Zou, M. Wang, X. Mu, N. Yang and L. Chen, Sens. Actuators, B, 2014, 192, 488–495 CrossRef CAS.
- Q. Hu, M. C. Paau, Y. Zhang, W. Chan, X. Gong, L. Zhang and M. M. F. Choi, J. Chromatogr. A, 2013, 1304, 234–240 CrossRef CAS PubMed.
- J. Bian, C. Huang, L. Wang, T. Hung, W. A. Daoud and R. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 4883–4890 CAS.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- Y. Song, S. Zhu, S. Xiang, X. Zhao, J. Zhang, H. Zhang, Y. Fu and B. Yang, Nanoscale, 2014, 6, 4676–4682 RSC.
- X. Hu, L. Cheng, N. Wang, L. Sun, W. Wang and W. Liu, RSC Adv., 2014, 4, 18818 RSC.
- Z. Yang, Z. Li, M. Xu, L. Zhang, J. Zhang, Y. Su, F. Gao and H. Wei, Micro Nano Lett., 2013, 5, 247–259 Search PubMed.
- Y. Dong, H. Pang, H. Bin Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 52, 7800–7804 CrossRef CAS PubMed.
- H. B. Yang, Y. Q. Dong, X. Wang, S. Y. Khoo and B. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 1092–1099 CAS.
- H. B. Yang, Y. Q. Dong, X. Wang, S. Y. Khoo, B. Liu and C. M. Li, Sol. Energy Mater. Sol. Cells, 2013, 117, 214–218 CrossRef CAS.
- M. J. Krysmann, A. Kelarakis, P. Dallas and E. P. Giannelis, J. Am. Chem. Soc., 2012, 134, 747–750 CrossRef CAS PubMed.
- X. Dong, Y. Su, H. Geng, Z. Li, C. Yang, X. Li and Y. Zhang, J. Mater. Chem. C, 2014, 2, 7477 RSC.
- Y. Dong, R. Wang, W. Tian, Y. Chi and G. Chen, RSC Adv., 2014, 4, 3701 CAS.
- X. Lin, G. Gao, L. Zheng, Y. Chi and G. Chen, Anal. Chem., 2014, 86, 1223–1228 CrossRef CAS PubMed.
- J. Liu, X. Liu, H. Luo and Y. Gao, RSC Adv., 2014, 4, 7648 RSC.
- Y. Dong, R. Wang, H. Li, J. Shao, Y. Chi, X. Lin and G. Chen, Carbon, 2012, 50, 2810–2815 CrossRef CAS.
- Y. Dong, R. Wang, G. Li, C. Chen, Y. Chi and G. Chen, Anal. Chem., 2012, 84, 6220–6224 CrossRef CAS PubMed.
- J. J. Liu, X. L. Zhang, Z.-X. Cong, Z.-T. Chen, H.-H. Yang and G.-N. Chen, Nanoscale, 2013, 5, 1810–1815 RSC.
- W. Shi, X. Li and H. Ma, Angew. Chem., Int. Ed., 2012, 51, 6432–6435 CrossRef CAS PubMed.
- F. Wang, Z. Xie, B. Zhang, Y. Liu, W. Yang and C. Liu, Nanoscale, 2014, 6, 3818–3823 RSC.
- S. Qu, X. Wang, Q. Lu, X. Liu and L. Wang, Angew. Chem., Int. Ed., 2012, 51, 12215–12218 CrossRef CAS PubMed.
- D. Qu, M. Zheng, L. Zhang, H. Zhao, Z. Xie, X. Jing, R. E. Haddad, H. Fan and Z. Sun, Sci. Rep., 2014, 4, 5294 CAS.
- J. Yang, Y. Zhang, S. Gautam, L. Liu, J. Dey, W. Chen, R. P. Mason, C. A. Serrano, K. A. Schug and L. Tang, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10086–10091 CrossRef CAS PubMed.
- Y. Zhang, R. T. Tran, I. S. Qattan, Y.-T. Tsai, L. Tang, C. Liu and J. Yang, Biomaterials, 2013, 34, 4048–4056 CrossRef CAS PubMed.
- D. Gyawali, S. Zhou, R. T. Tran, Y. Zhang, C. Liu, X. Bai and J. Yang, Adv. Healthcare Mater., 2014, 3, 182–186 CrossRef CAS PubMed.
- A. S. Wadajkar, T. Kadapure, Y. Zhang, W. Cui, K. T. Nguyen and J. Yang, Adv. Healthcare Mater., 2012, 1, 450–456 CrossRef CAS PubMed.
- W. Kasprzyk, S. Bednarz and D. Bogdał, Chem. Commun., 2013, 49, 6445–6447 RSC.
- H. Zhou, X. Lv, L. Zhang, A. Gong, A. Wu, Z. Liang, G. Peng and H. Lin, J. Mater. Chem. C, 2014, 2, 9625–9630 RSC.
- M. B. Smith and J. March, March's Advanced Organic Chemistry, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2006 Search PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer-Verlag, 3rd edn, 2006 Search PubMed.
- M. Rida, H. El Meslouhi, N.-E. Es-Safi, E. M. Essassi and J. Banoub, Rapid Commun. Mass Spectrom., 2008, 22, 2253–2268 CrossRef CAS PubMed.
- T. Kind and O. Fiehn, Bioanal. Rev., 2010, 23–60 CrossRef PubMed.
- M. Zheng, Z. Xie, D. Qu, D. Li, P. Du, X. Jing and Z. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 13242–13247 CAS.
- X. Liu, N. Zhang, T. Bing and D. Shangguan, Anal. Chem., 2014, 86, 2289–2296 CrossRef CAS PubMed.
- F. Wang, Z. Xie, H. Zhang, C. Liu and Y. Zhang, Adv. Funct. Mater., 2011, 21, 1027–1031 CrossRef CAS.
- F. Wang, Z. Xie and C. Liu, New J. Chem., 2014, 38, 1601 RSC.
- T. H. Kim, F. Wang, P. McCormick, L. Wang, C. Brown and Q. Li, J. Lumin., 2014, 154, 1–7 CrossRef CAS.
- J. Tang, Y. Zhang, B. Kong, Y. Wang, P. Da, J. Li, A. a Elzatahry, D. Zhao, X. Gong and G. Zheng, Nano Lett., 2014, 14(5), 2702–2708 CrossRef CAS PubMed.
- Y. Wang, S. Kalytchuk, Y. Zhang, H. Shi, S. V. Kershaw and A. L. Rogach, J. Phys. Chem. Lett., 2014, 5, 1412–1420 CrossRef CAS PubMed.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, M. Karakassides and E. P. Giannelis, Small, 2008, 4, 455–458 CrossRef CAS PubMed.
- F. Du, Y. Min, F. Zeng, C. Yu and S. Wu, Small, 2014, 10, 964–972 CrossRef CAS PubMed.
- C. M. Comisar, S. E. Hunter, A. Walton and P. E. Savage, Ind. Eng. Chem. Res., 2008, 577–584 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03226a |
| This journal is © The Royal Society of Chemistry 2015 |