Recent advances in f-element separations based on a new method for the production of pentavalent americium in acidic solution
Abstract
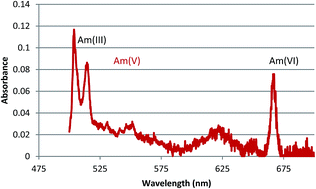
The peroxydisulfate anion has long been used for the preparation of hexavalent americium (AmVI) from the normally stable AmIII valence state in mildly acidic solutions. However, there has been no satisfactory means to directly prepare the pentavalent state (AmV) in that medium. Some early literature reports indicated that the peroxydisulfate oxidation was incomplete, and silver ion catalysis in conjunction with peroxydisulfate became accepted as the means to ensure quantitative generation of AmVI. Incomplete oxidation would be expected to leave residual AmIII, or to produce AmV in treated solutions. However, until recently, the use of peroxydisulfate as an AmV reagent has not been reported. Here, parameters influencing the oxidation were investigated, including peroxydisulfate and acid concentration, temperature, duration of oxidative treatment, and the presence of higher concentrations of other redox active metals such as plutonium. Using optimized conditions determined here, quantitative AmV was prepared in an acidic solution and the UV/Vis extinction coefficients of the AmV 513 nm peak were measured over a range of nitric acid concentrations. The utility of AmV for separations from the lanthanides and curium by solvent extraction, organic column chromatography and inorganic ion exchangers was also investigated.
- This article is part of the themed collection: RSC Advances Editors' collection: f Block Chemistry

 Please wait while we load your content...
Please wait while we load your content...