Synthesis, characterization and environmental application of silica grafted photoactive substances isolated from urban biowaste
Maria Luisa Testa*a,
Maria Laura Tumminoab,
Silvia Agostinib,
Paola Avettab,
Francesca Deganelloa,
Enzo Montoneric,
Giuliana Magnaccabd and
Alessandra Bianco Prevot*b
aCNR-ISMN, via U. La Malfa 153, I-90146, Palermo, Italy. E-mail: marialuisa.testa@cnr.it; marialuisa.testa@ismn.cnr.it; Fax: +390916809399; Tel: +390916809253
bUniversità di Torino, Dipartimento di chimica, Italy
cBiowaste Processing, Via XXIV Maggio 25, I-37126 Verona, Italy
dNIS Centre of Excellence, via P. Giuria 7, I-10125 Torino, Italy. E-mail: alessandra.biancoprevot@unito.it; Fax: +390116705242; Tel: +390116707631
First published on 30th April 2015
Abstract
A waste-derived photoactive substance sourced from the green fraction of urban refuses (CVT230) was immobilized on different types of silica support, one amorphous and the other two with controlled porosity (HMS and SBA). In this fashion, three hybrid systems were obtained, which contained CVT230 as an insoluble photoactive component. They were tested in the photodegradation of 4-methylphenol in aqueous solution irradiated by simulated solar light and results showed that they are able to promote the total photodegradation of the pollutant. The materials, both before and after irradiation, were characterized by high-resolution transmission electron microscopy (TEM), small angle X-ray scattering (SAXS), N2 gas-volumetric adsorption, infrared spectroscopy (FTIR) and thermogravimetric analysis (TGA). The SBA silica hybrid system showed the best performance in terms of stability and reusability, after multiple irradiation cycles. This behaviour can be correlated to the silica morphology and texture, being capable to better allocate and stabilize the CVT230 molecules.
Introduction
Over the last few years, a main cause for concern has been the environmental damage provoked by waste accumulation and by soil, water and air pollution. Proper recycling and reusing of wastes has become mandatory for the sustainable development of our society. In this context, technological development of both urban biowaste (UBW) processing and products for environmental remediation is an important societal challenge. Recently, several papers have reported that the recalcitrant lignin-like fraction of fermented UBW is a cost effective source of soluble bio-based substances (SBO) which can be applied in disparate fields.1,2 Depending on the origin and treatment of the UBW, different SBO can be obtained, showing a wide range of chemical compositions and properties. These substances can be considered as supramolecular assemblies with 76–463 kD weight average molecular weight. They contain long aliphatic chains, aromatic rings and several functional groups such as carboxyl, primary and substituted amine and amide, carbonyl, hydroxyl, phenol, ether or ester. SBO have been reported to bear chemical similarities with humic substances, and to exhibit surfactant behaviour1 and photosensitizing properties.3 Photosensitizers have been demonstrated to be quite effective in enhancing the photodegradation of water pollutants and promoting significant mineralization of the organic carbon. Interestingly, other workers2 have shown that the same SBO applied to soil enhances the plant photosynthetic activity. These authors, discussing their results on plant photosynthesis in comparison with those published on the photochemical degradation of organic pollutants in industrial effluent,3 have proposed that SBO might promote either carbon fixation or mineralization, according to the different operational environments. The proposed scenario certainly adds further worthwhile fascinating scope to continue investigating and/or to optimize the performance of SBO as photosensitizers.Most of the published papers on the photosensitizing properties of SBO deal with a particular type of product (CVT230) was isolated from urban public park trimming and home gardening residues aged, under aerobic conditions, for 230 days. This product has been tested as a homogenous photosensitizer for the degradation of chlorophenols, which are toxic and non-biodegradable compounds found in wastewater, groundwater and soil.3 The proposed mechanism for SBO photoactivity is based on the mechanisms previously proposed for natural humic substances. It is based on the production, under UV-vis irradiation, of excited triplet states that can in turn react with the organic substrate by two main mechanisms: hydrogen-transfer and energy-transfer.4,5 In a previous work,6 concerning the photosensitizing effect of a group of SBO for dye degradation, hydrogen-transfer was hypothesized as the main mechanism; i.e., generated hydrogen atoms can be transferred to the dissolved oxygen with the formation of various reactive oxygenated species, particularly ˙OH and 1O2, which in turn contribute to the degradation of the probe molecule. A further study was devoted to confirm this hypothesis using 4-chlorophenol (4-CP) as the probe molecule.7 The reported results show a complete detoxification of the system, encouraging deeper investigation on SBO activity. One of the most important drawbacks of using CVT230 as the photoactive material is its solubility in water and its consequent difficulty to be separated, after the treatment, from the reaction system. On the contrary, the recovery of an insoluble material is easier, less expensive and more eco-friendly. The present research aimed to turn the soluble photoactive CVT230 into an insoluble form by immobilizing it within a suitable water-insoluble support. This approach is expected to yield a stable and recyclable material for performing repeated wastewater photodegradation cycles. This perspective offers a considerable advantage for the engineering of remediation systems operating in a real environment. To achieve this objective, the similarity between SBO and humic substances was considered. Actually, several synthetic routes have been studied for supporting humic acids (HA) onto silica surfaces in order to exploit the sorbent properties of the hybrid system.8–11 Depending on the structure of HA, different functional groups were used for the chemical immobilization. Guczi et al.12 used the phenolic groups of HA as linkers, although the reactions for the activation of the silica support were troublesome. Moreover, the alcoholic groups of HA were grafted onto epoxypropyl silica, while the amino groups of HA reacted with the chloromethylated support.13
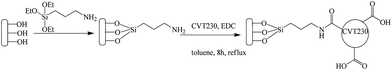
In the present research, the presence of carboxylic moieties in CVT230 was exploited for the preparation of the heterogeneous hybrid system CVT230/SiO2 (see Scheme 1).
In order to study the role of the silica morphology and textural properties on the photoactivity, CVT230 was grafted onto three different types of silica, one amorphous (Am) and the other two with controlled mesoporosity (HMS and SBA-15). The obtained hybrid organic–inorganic materials were then characterized by TEM microscopy, small angle X-ray scattering, N2 gas-volumetric adsorption, IR spectroscopy and thermogravimetric analysis. The photosensitizing activity, the reusability after one reaction cycle and the stability of the hybrid organic–inorganic materials after several irradiation runs were evaluated using the degradation of 4-methylphenol (4-MP) in aqueous solution under simulated solar light irradiation as the probe process.
Experimental
Characterization of CVT230
CVT230 was obtained and characterized as previously reported.2,14 It was sourced from a compost of urban waste and park trimming residues aged for 230 days. This material was hydrolyzed with KOH alkaline water at pH 13 and 60 °C to yield a soluble hydrolysate and insoluble matter. The former was run through an ultra filtration polysulphone membrane with 5 kD cut off. The membrane retentate was dried at 60 °C to yield the final SBO product as a black solid in a 15–20% yield, relative to the starting material. The product had the following C mole% composition: aliphatic C (29.05%); aromatic C (16.03%); anomeric C (6.73%); ketonic C (2.13%); phenolic groups (8.17%); carboxylic acids and amides (11.75%); O-alkyl groups (20.18%); and N-alkyl (5.96%). The FTIR spectrum related to the product contained bands at the following wavenumbers: 3360 cm−1 (broad, due to OH and NH stretching modes); 2928 cm−1 (complex, due to C–H stretching vibration); 1715 cm−1 (strong, due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of COOH groups); 1626 cm−1 (relative to conjugated C
O stretching of COOH groups); 1626 cm−1 (relative to conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups); 1565 and 1420–1380 cm−1 (due to OCO symmetric and asymmetric stretching signals); and 1225–1033 cm−1 (due to
C groups); 1565 and 1420–1380 cm−1 (due to OCO symmetric and asymmetric stretching signals); and 1225–1033 cm−1 (due to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, C–O–C stretching modes). The composition of the CVT230 mineral fraction, after calcination in a muffle furnace was determined by ICP analysis and it is expressed as % w/w: Si (2.55 ± 0.01); Fe (0.77 ± 0.04); Al (0.49 ± 0.04); Mg (1.13 ± 0.06); Ca (6.07 ± 0.38); K (3.59 ± 0.21); and Na (0.16 ± 0.01).
CH, C–O–C stretching modes). The composition of the CVT230 mineral fraction, after calcination in a muffle furnace was determined by ICP analysis and it is expressed as % w/w: Si (2.55 ± 0.01); Fe (0.77 ± 0.04); Al (0.49 ± 0.04); Mg (1.13 ± 0.06); Ca (6.07 ± 0.38); K (3.59 ± 0.21); and Na (0.16 ± 0.01).
Sample preparation
For the preparation of the hybrid CVT230/SiO2 samples a stepwise approach, represented in Scheme 1, was used. First, the silica support was synthesized and the surface was functionalized with aminopropyl groups. Afterwards, the covalent immobilization of CVT230 on aminopropylsilica (AMPS) was carried out using a published method8 which led to amide bond formation by the reaction of AMPS amino groups with the CVT230 carboxylic groups activated by N-(3-dimethylamino propyl)–N′-ethylcarbodiimide hydrochloride (EDC).All the compounds used in the synthesis were purchased from commercial suppliers (Aldrich, Fluka) and used without further purification. Distilled toluene and dimethylformamide (DMF) were stored on molecular sieves (4 Å) to assure their complete dryness.
Synthesis of the silica support
Different types of silica, hereinafter referred to as Am, HMS and SBA, were synthesized by sol–gel techniques according to published procedures.15–17To obtain the Am, tetraethoxysilane (TEOS, 30 mL, 0.135 mol) was dissolved in ethanol (20 mL) and stirred at 45 °C for 15 minutes. Then, 19 mL of an aqueous solution of acetic acid at pH 5 was added to the mixture. The temperature was increased to 80 °C until the formation of the gel was achieved. The obtained wet gel was dried at 110 °C overnight and then calcined in air at 450 °C for 4 h.
For the synthesis of HMS, TEOS (12 mL, 0.054 mol) was added to a stirred solution of ethanol (31 mL), water (31 mL) and 1-dodecylamine (3.68 mL, 0.016 mol). The mixture was stirred at room temperature for 24 h. The white precipitate obtained was filtered under vacuum and washed with deionized water (36 mL) and ethanol (36 mL). The solid was dried at room temperature overnight and then calcined in air at 600 °C for 4 h.
The SBA-15 was prepared using a non-ionic amphiphilic triblock polymer, Pluronic P123, as a surfactant. The polymer (8.1 g) was dissolved in deionized water (146.8 mL) and HCl conc. (37%, 4.4 g) and stirred overnight at 35 °C in a 250 mL one neck flask. TEOS (17.1 mL, 0.077 mol) was quickly added to this solution and stirred for 24 h at 35 °C. The milky suspension was aged at 100 °C for 24 h in a closed polypropylene bottle. The solid product was filtered, washed with a HCl/water mixture and calcined in air at 500 °C for 5 h (heating ramp of 1 °C min−1).
Functionalization of silica with AMPS
The amorphous, HMS and SBA silicas were functionalized with aminopropyl groups by adopting a grafting method reported in the literature.16 In a typical procedure, a mixture of 1.00 g of calcined silica in 15.0 mL of dry toluene and 1.00 mL of 3-aminopropyltriethoxysilane (AMPS) was refluxed for 24 h. The material was filtrated in vacuo, washed with toluene and dried overnight at 85 °C. The amorphous and the mesoporous grafted silica samples were labelled as NH2-Am, NH2-HMS and NH2-SBA.Covalent immobilization of CVT230 onto silica
For the immobilization of CVT230 onto silica a procedure used for supporting HA on silica was adopted.11 CVT230 (435 mg) and EDC (100 mg) were added to a suspension of 1.0 g of amino-functionalized silica in 50 mL of toluene. The mixture was refluxed for 12 h. The material was filtered under vacuum and washed several times with toluene, ethanol and sodium chloride solution (1 M). In order to end-cap the free amino groups and to obtain a stable product, the material was then suspended in 50 mL of DMF containing 5% acetic anhydride and stirred at room temperature overnight. The product was washed with DMF, H2O and acetone and finally dried at 85 °C overnight. The immobilized CVT230 materials were labelled as CVT230-Am, CVT230-HMS and CVT230-SBA.In order to roughly evaluate the stability of the organic–inorganic hybrid material before its use in photochemical tests, 200 mg of CVT230-Am was suspended in 10 mL of water. The suspension was stirred at room temperature for 8 h and no evidence of CVT230 leaching was found in the clear aqueous solution. The material was then filtered under vacuum and dried at 100 °C overnight. It was recovered as a dry solid in 98.5% yield. The 1.5% loss was likely due to the filtration procedure.
Material characterization
High-Resolution Transmission Electron Microscopy (TEM) was performed using a JEOL JEM 3010UHR (300 kV) instrument fitted with a single crystal LaB6 filament. The sample was dry-deposited on Cu “holey” carbon grids (200 mesh).Small angle X-ray Scattering (SAXS) patterns were measured with a Bruker vertical goniometer using Ni-filtered Cu Kα radiation in order to verify the expected ordered porous structure of the SBA-15 and CVT230-SBA systems. A proportional counter and 0.05° step sizes in 2θ were used.
The textural properties of the samples outgassed at 60 °C for several hours (in order to obtain the standard residual pressure of 10−2 mbar in the outgassing system) were obtained using the gas-volumetric apparatus ASAP2020 by Micromeritics. The BET method in the standard relative pressure range 0.06–0.2p/p0 allowed the specific surface areas of the samples before and after the functionalization procedure to be obtained. The total pore volume, Vp, and the pore size distribution were evaluated by applying the DFT model for slit (Am and HMS samples) and cylinder (SBA sample) pores using a low regularization.
FTIR spectra were recorded on a Jasco FTIR 5300 spectrophotometer at a resolution of 4 cm−1, accumulating 100 scans for each spectrum. Self supporting pellets were prepared with 1 mg of sample and 160 mg of KBr, pressing the mixture at 2 ton for 2 min.
Thermogravimetric analyses (TGA) were performed with a TA Instruments TGA 2050 Thermo-Gravimetrical Analyzer in order to assess the amount of organic species bonded to the siliceous supports. Analyses were performed on powdery samples under air flow and using a heating ramp of 10 °C min−1 from 30 to 800 °C. The measurements were performed in aluminium oxide sample holders.
Degradation of 4-methylphenol
Preliminary experiments were performed on the aqueous solutions of 4-MP at 10 mg L−1, irradiated for 5 and 8 hours in the absence of silica-CVT230, in order to identify the direct photolysis of the substrate. The results showed that the photolysis was not relevant (about 5–7%) compared to the degradation attained in the presence of the CVT230-silica hybrid materials as evaluated by HPLC measurements of 4-MP. Absorption tests in the dark were also performed by stirring an aqueous suspension of 800 mg L−1 of silica-SBO and 4-MP (10 mg L−1) for 4 and 8 hours and then filtering the suspension and analyzing the 4-MP left in solution. The absorbed 4-MP amount can be considered to be negligible (2–4%). Afterwards, 4-MP photodegradation was studied in the presence of the CVT230-silica hybrid materials. Aqueous suspensions of the functionalized silica samples CVT230-Am, CVT230-HMS and CVT230-SBA in the concentration range from 80 up to 1200 mg L−1 were prepared with ultrapure water (Millipore Milli-Q™ system) and stirred for 30 min before the irradiation. The initial concentration of 4-MP (>99% Aldrich) was kept constant in all the irradiated suspensions and was 10 mg L−1. The photodegradation trials were carried out by irradiating, under continuous stirring, 5 mL of the suspensions in a closed Pyrex® cell with a xenon (1500 W) lamp (Solarbox, CO.FO.Megra-Mi) equipped with a 340 nm cut-off filter. The irradiance of the lamp, measured with a UV-Multimeter system, was 26 W m−2. After irradiation, in order to remove the photoactive material, the samples were centrifuged and filtered through a cellulose acetate 0.45 μm pore diameter filter (Millipore).Degradation of 4-MP was followed by HPLC, employing a Merck-Hitachi instrument, equipped with a Lichrospher RP-C18 column (125 mm × 4 mm i.d., d.p. 5 μm, from Merck), a Rheodyne injector, L-6200 pumps and a UV-vis Merck Hitachi L-4200 detector and D-7000 HPLC System Manager (HSM) software. 4-MP was eluted employing isocratic conditions, with water (70%) and acetonitrile (30%). The flow rate was 1.0 mL min−1, the retention time was 5.0 minutes and the detector wavelength was 220 nm.
In order to assess the possibility of re-using the CVT230-silica samples the following procedure was adopted: after an irradiation time corresponding to the complete disappearance of 4-MP (15 hours, as reported below in the Results and discussion section), the suspension was centrifuged and the solid was separated, then washed and centrifuged several times. The resulting solid was dried at 50 °C for two days and reused.
A different procedure was chosen in order to age the functionalized silica samples and to evaluate any change in the hybrid materials. A suspension (50 mL) of the functionalized silicas (1000 mg L−1) and 4-MP (10 mg L−1) was irradiated in a Solarbox for 15 hours. Afterwards, in the same cell, 4-MP (10 mg L−1) was added and again irradiated in the same conditions. This procedure was repeated for a total irradiation time of 45 hours. After each step, the complete abatement of the substrate was verified. At the end of the treatment the samples were recovered, centrifuged, washed with a few milliliters of water two times, dried at 50 °C for two days and characterized. Samples will be indicated as aged (suffix a) in text and figures. For example, CVT230-Am-a indicates the amorphous silica functionalized with CVT230 after 45 hours irradiation in the presence of 4-MP. Organic matter leaching was checked, analyzing the aqueous phase after the separation of the functionalized silicas, by UV spectroscopy using a double beam UV-vis spectrophotometer CARY 100 SCAN-VARIAN with a quartz cell of 1 cm path length.
Results and discussion
Material characterization
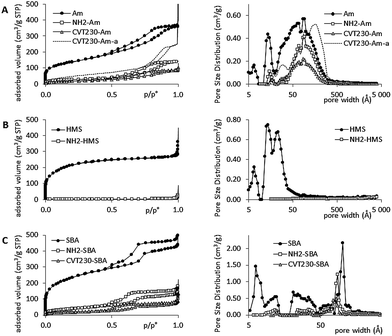
TEM images are reported in Fig. 1 with the best magnification useful to evidence the interesting features of the bare siliceous supports and the fresh and aged hybrid materials. The amorphous silica appears as aggregates of amorphous nanometric particles. After the functionalization with CVT230 some evidence of the presence of the amorphous organic phase can be seen as an undefined halo covering the particles and increasing their aggregation. After the aging of sample, no significant modification of the powder morphology occurred.The HMS silica is made of almost spherical particles of 150–400 nm diameter with a pineapple structure evidencing the radial porosity of the system. After functionalization with CVT230, the pineapple structure is completely lost, probably because the organic phase covers the particle surface and fills the pores. After the photodegradation reactions some structural damages are evident as the material edges are not regular anymore and small amorphous pendants formed around the particles (see arrows in Fig. 1 image C′).
The SBA silica is made of irregularly shaped particles where the regular structure of the mesopores appears clearly. The functionalization and the following ageing do not cause any modification in the microscopic appearance of the sample. No changes are visible and the hexagonal structure of the pores is maintained, as evidenced by image C′′ of Fig. 1.
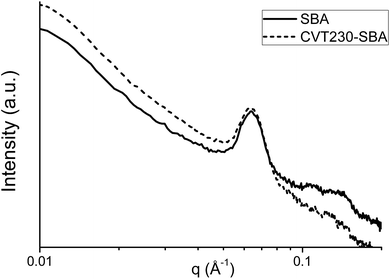
The SAXS patterns of the SBA support and CVT230-SBA are compared in Fig. 2. SAXS was applied to the bare sample, with the aim to evidence the order in the pore microstructure, and to the functionalized one with the intention to evaluate the effect of CVT230 immobilization on the preservation of the microstructural order. As expected, the SBA diffractogram shows signals at ∼0.90 (100), 1.50 (110) and 2.00 (200)° 2θ, attributable to the hexagonal geometry of the cylindrical pores. On the other hand, the CVT230-SBA pattern shows a well-defined signal at 0.90° 2θ, whereas the other two peaks are less intense, indicating a partial loss of the long range microstructural order. Nevertheless, the fact that the signal position remains the same confirms that the porous structure was not modified by the immobilization procedure of CVT230 onto SBA.
The data obtained by N2 gas-volumetric adsorption are collected in Table 1 and Fig. 3 for the bare silicas and the corresponding aminopropyl and CVT230-functionalized powders.
| Sample | BET surface area (m2 g−1) | DFT pore volume (cm3 g−1) | |
|---|---|---|---|
| <20 Å | >20 Å | ||
| Am | 497 | 0.12 | 0.38 |
| NH2-Am | 98 | 0.01 | 0.20 |
| CVT230-Am | 66 | <0.01 | 0.13 |
| CVT230-Am-a | 185 | 0.01 | 0.35 |
| HMS | 784 | 0.23 | 0.15 |
| NH2-HMS | ∼7 | Not measurable | Not measurable |
| CVT230-HMS | ∼5 | Not measurable | Not measurable |
| CVT230-HMS-a | ∼250 | Not measurable | Not measurable |
| SBA | 845 | 0.40 | 0.20 |
| NH2-SBA | 147 | 0.02 | 0.15 |
| CVT230-SBA | 70 | <0.01 | 0.09 |
| CVT230-SBA-a | ∼300 | Not measurable | Not measurable |
Unexpectedly, the amorphous (Am) material presented porosity in the micro and meso-range (<20 Å and >20 Å width, respectively). This porosity is not due to the presence of pores inside the particles (the particles are very small if compared with the porosity observed), but is assignable to the aggregation of particles and therefore to interparticle void spaces. HMS showed the typical mesoporosity expected for this material, i.e. pores of 20–30 Å diameter18 and some micropores with diameters less than 20 Å. Ordered mesopores visible at ∼100 Å were evidenced in the SBA sample together with a complex distribution of pores (essentially micropores and small mesopores).19
As expected, in all cases the grafted samples showed a decrease in surface area and porosity, already visible after aminopropyl functionalization and much more evident after CVT230 immobilization. The effect was dramatic in the case of the HMS sample, resulting in the occlusion of all the pores after the aminopropyl functionalization procedure. Whereas, the Am and SBA samples, with different families of pores, showed a selective effect due to the grafting of aminopropyl groups and CVT230 macromolecules. The weight average molecular weight of CVT230 was estimated to be 70–80 kD.1 In the materials containing the grafted CVT230, all the small pores disappeared and the larger ones decreased both in amount and size. In the case of the highly ordered SBA sample, the pore diameters decreased from 100 to 90 Å (after aminopropyl insertion) and then to 80 Å (after CVT230 immobilization). These results suggest that the functionalization occludes the small pores (i.e., pores smaller than 50 Å width), whereas the larger pores are only partially occupied by the grafting molecules.
The morphology of the aged samples was analyzed using N2 adsorption. The small amount of sample recovered after the photodegradation cycles limits the accuracy of the data, but we preferred to perform all the analyses on the same starting dose of sample, instead of introducing uncertainties by mixing powders coming from different batches. Nevertheless, in all cases it was possible to observe an increase in the specific surface area. In the case of the CVT230-Am-a sample, porosity measurements evidenced an increase in pore amount and size with respect to CVT230-Am. These data suggest that a partial loss of the organic phase occurred during the photodegradation reaction.
The FTIR spectra of pure amorphous silica and its derivatives NH2-Am and CVT230-Am are shown in Fig. 4. In all spectra the strong peak at 1100 cm−1 is assigned to the Si–O–Si asymmetric stretching vibration, while the peaks at 850 and 500 cm−1 are characteristic of the Si–OH bending from silica itself. In the silica spectrum, the weak signal at 1630 cm−1 is typical of the bending vibration of the H–O–H bonds (δHOH) in physisorbed molecular water, while the broad band at 3500–3600 cm−1 arises from the stretching vibration of OH groups interacting via H-bonding. When the aminopropyl groups are grafted onto the silica surface, the OH group signals decrease and the NH2 group signals appear at ∼3400 cm−1. Moreover, the immobilization of CVT230 onto the silica support was confirmed by the appearance of a strong new peak at 1635 cm−1, typical of the amide group formed by the reaction between the NH2-silica amino groups and the CVT230 carboxylic groups. There are no relevant differences among the spectra of the hybrid organic–inorganic samples derived from the different types of silica (Am, HMS and SBA).
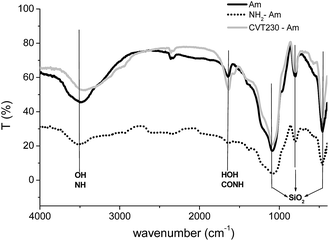 | ||
| Fig. 4 FTIR spectra of the amorphous pure silica (Am, black line), doped with aminopropyl groups (NH2-Am, dash dot) and after CVT230 grafting (CVT230-Am, grey line). | ||
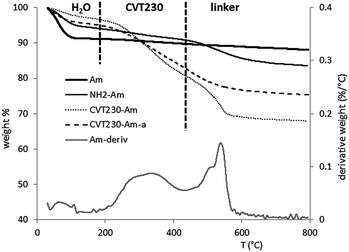
All the samples (silicas, aminopropyl-silicas and the CVT230-functionalized materials) were analyzed by means of thermogravimetric analysis in order to quantify the amount of organic species bonded during the grafting procedure. As an example, Fig. 5 reports the TGA profiles relating to the Am sample in the three steps of preparation (bare silica, aminopropyl silica and the CVT230-functionalized sample). Also the profile of the aged material is reported for the sake of comparison. The amount of water and organic species released, evaluated for all the samples, is reported in Table 2.
| Sample | Water release (%) (30–190 °C) | Organic release (%) | ||
|---|---|---|---|---|
| Total amount | Linker (430–800 °C) | CVT230 (190–430 °C) | ||
| Am | 9 (+3) | — | — | — |
| NH2-Am | 6 | 11 | 11 | — |
| CVT230-Am | 3 | 29 | 11 | 18 |
| CVT230-Am-a | 5 | 20 | 7 | 13 |
| HMS | 21 (+3) | — | — | — |
| NH2-HMS | 8 | 15 | 15 | — |
| CVT230-HMS | 8 | 27 | 12 | 15 |
| CVT230-HMS-a | 9 | 29 | 12 | 17 |
| SBA | 6 (+4) | — | — | — |
| NH2-SBA | 7 | 13 | 13 | — |
| CVT230-SBA | 7 | 27 | 9 | 18 |
| CVT230-SBA-a | 4 | 26 | 9 | 17 |
Pure silica presents a main weight loss step in the 30–190 °C range and a second slight weight decrease from 190 up to 800 °C. The first step is certainly due to the loss of physisorbed water, whereas the second weight loss step should be due to the release of water formed from surface OH group condensation. All the bare silica systems show similar trends except the HMS material which presents a relevant amount of water released indicating an important hydrophilic behavior of the system.
NH2-silica displays two clearly visible steps: the first one in the range 30–190 °C, due to the release of water, and the second one, centered at about 500 °C, due to the thermal degradation of organic matter. In the first step, where a more limited amount of water is released with respect to the bare system, the data in Table 2 are consistent with the lower hydrophilicity of the HMS system as a result of the aminopropyl functionalization. The second step allows the determination of the amount of aminopropyl groups (linkers) present on the surface of the systems and can evidence a slightly higher functionalization degree for the NH2-HMS system (15% of organic with respect to 13 and 11% for the SBA and amorphous silica systems, respectively).
The CVT230-functionalized systems (see Fig. 5) show a two-step trend: the first step in the range 30–190 °C is due to water loss. The second step is continuous over the entire 190–800 °C range, where it is possible to notice the contribution due to CVT230 loss (190–430 °C) and aminopropyl elimination (430–800 °C). The amounts relating to these two contributions and the total amount of organic species released by the materials are reported in Table 2. Lastly, the analysis of the aged samples after the photoreaction indicate three different situations: (i) the Am sample reflects an important loss of the active phase (ca. 28% of the total amount of CVT230 immobilized on the support is lost); (ii) the HMS sample shows an apparent increase in CVT230 amount. There are no logical reasons explaining the increase in the amount of organic species after the ageing procedure. Nevertheless, TEM results suggest that the sample might have been modified in such a way that a comparison between the TGA curves before and after prolonged irradiation and contact with 4-MP becomes impossible; (iii) the SBA turns out to be the more stable hybrid material with a negligible loss of the active phase after the ageing procedure. This fact could be attributed to the morphology and texture of the SBA support, that allows a better distribution of organic linkers and, as a consequence, of the photoactive CVT230 on the support surface. A similar effect of the SBA has been reported in the case of an acid linker containing propyl-sulfonic acid functional groups immobilized onto SBA, HMS and amorphous silica.20 In that case the SBA hybrid system showed the best performance, due to a better distribution of active sites not only in terms of the total amount of linkers but also the surface density.20 SBA silica is thus less susceptible to modifications than the other two silica supports, being capable to better allocate organic molecules in its organized porosity, without losing the original microstructural order.
Photodegradation activity
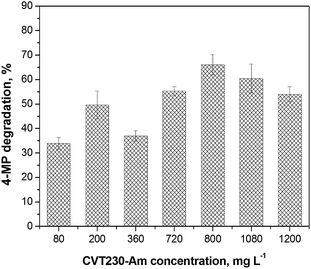
CVT230-Am, CVT230-HMS and CVT230-SBA aqueous suspensions were tested for the photodegradation of 4-MP in a water solution. The effects of the amount of CVT230-silica, the irradiation time and the CVT230-silica recycling were studied. First of all the 4-MP photodegradation was performed during 6 h runs for all three CVT230-silica samples varying their concentrations in the range 80–1200 mg L−1. This was the result of previous trials indicating 6 hours as a reasonable reaction time.Fig. 6 reports the percentage of 4-MP degradation versus the CVT230-Am concentration. The degradation efficiency increases with the photoactive material concentration up to about 800 mg L−1 and then becomes almost constant within the limits of the experimental uncertainty. Analogous trends were also observed for CVT230-HMS and CVT230-SBA, hence 800 mg L−1 was chosen as the optimal concentration in order to achieve a good level of degradation without useless excess of the hybrid material. Fig. 7 shows the kinetic profiles for all the samples during an 8 h irradiation time. Moreover, investigations on the adsorption of 4-MP with the CVT230-silicas and on 4-MP direct photolysis in pure aqueous solution were carried out. No adsorption and no photolysis were evidenced (see Fig. 7). Very similar photodegradation behaviors were observed for the three hybrid materials showing an abatement of about 70–80% of 4-MP after 8 h irradiation. The complete 4-MP disappearance was obtained for all the samples after 15 hours of irradiation. This is consistent with the fact that all samples contained the same amount of the grafted CVT230 (Table 2). The results indicate that the photodegradation of 4-MP mainly depends on the photoactive CVT230 immobilized on the silica surface. Hence the degradation could be driven mainly by the photogeneration of reactive oxygenated species rather than by other mechanisms, implying substrate adsorption on the CVT230-silica surface. The fact that the activity is directly related to the CVT230 phase, and not to other adsorption phenomena involving the support, can be confirmed observing the effects of a surface area normalization on the degradation activity shown by the three materials. All systems show similar catalytic activity for a mass of catalyst, but CVT230-Am and CVT230-SBA possess specific surface areas of about 70 m2 g−1, whereas CVT230-HMS shows a specific surface area which is at least 10 times lower than the others. If a normalization is carried out for an area of catalyst, one square meter of the HMS-based system shows a reactivity which is at least ten times higher than the others. This is possible only considering that the Am-based and SBA-based systems expose not only the active CVT230 phase, but also the inactive siliceous support to the substrate, whereas the HMS-based system exposes much more of the active phase to the reaction, i.e. an amount at least ten times higher.
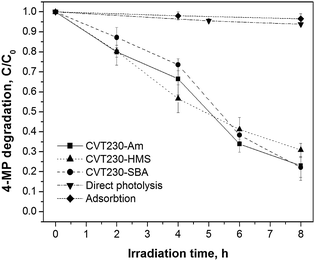 | ||
| Fig. 7 Degradation of 4-MP (10 mg L−1) versus irradiation time in the presence of the amorphous, HMS and SBA based systems (800 mg L−1). The adsorption and photolysis profiles are also shown. | ||
The possibility of re-using the material after its recovery and resuspension was tested on CVT230-Am: after 6 hours of irradiation, the results evidenced that the photoactivity was maintained. To assess the effect of the ageing of the photoactive material by longer irradiation, three cycles of 15 hours of irradiation in the presence of an initial concentration of 10 mg L−1 of 4-MP for each cycle were carried out. The abatement of 4-MP was complete in all the examined cases. At the end of the third run the material was separated and the UV-vis spectrum of the solution was recorded (see Fig. 8). It can be observed that the absorbance is not negligible. This fact may be caused by the partial detachment of the CVT230 material from the silica support.
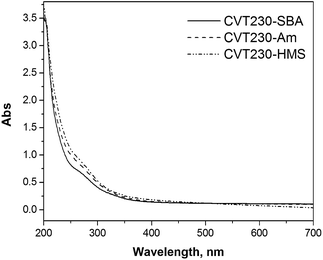 | ||
| Fig. 8 UV-vis spectra of the aqueous solutions obtained after the separation of the three photoactive materials irradiated in the presence of 4-MP for three cycles (total irradiation time = 45 h). | ||
Conclusions
For the first time a photoactive bio-based substance (CVT230) was grafted onto different silica supports and tested as a heterogeneous photosensitizer for the abatement of 4-methylphenol. All materials were able to completely promote the photodegradation of 4-methylphenol. Despite a slight depletion of the organic component, the photoactive performance of the CVT230-silica was not reduced after 15 hours of irradiation, as confirmed upon re-using the material for a second 4-MP degradation trial. The results evidence that the best performance and stability was achieved with the SBA hybrid material. This is most likely due to the particular morphology and texture of SBA, which best hosts and protects the CVT230 from self-degradation and leaching.Acknowledgements
The authors are thankful to 7thFP IRSES-2010-269128-EnvironBos Marie Curie Action for funding. Studio Chiono ed Associati in Rivarolo Canavese (TO) and Acea Pinerolese Spa in Pinerolo (TO) are also greatly acknowledged, for supplying the CVT230 and its sourcing material respectively. The scientific activities described in this paper belong to project MAT4TREAT: it has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 645551. The authors thank Dr Francesco Giordano for the SAXS measurements.Notes and references
- E. Montoneri, D. Mainero, V. Boffa, D. G. Perrone and C. Montoneri, Int. J. Global Environ., 2011, 11, 170–196 CrossRef.
- O. Sortino, E. Montoneri, C. Patanè, R. Rosato, S. Tabasso and M. Ginepro, Sci. Total Environ., 2014, 487C, 443–451 CrossRef PubMed.
- (a) P. Avetta, F. Bella, A. Bianco Prevot, E. Laurenti, E. Montoneri, A. Arques and L. Carlos, ACS Sustainable Chem. Eng., 2013, 1, 1545–1550 CrossRef CAS; (b) J. Gomis, A. Bianco Prevot, E. Montoneri, M. C. Gonzalez, A. M. Amat, D. O. Martire, A. Arques and L. Carlos, Chem. Eng. J., 2014, 235, 236–243 CrossRef CAS.
- A. Amine-Khodja, O. Trubetskaya, O. Trubetskoj, L. Cavani, C. Ciavatta, G. Guyot and C. Richard, Chemosphere, 2006, 62, 1021–1027 CrossRef CAS PubMed.
- C. Richard, D. Vialaton, J.-P. Aguer and F. Andreux, J. Photochem. Photobiol., A, 1997, 111, 265–271 CrossRef.
- A. Bianco Prevot, D. Fabbri, E. Pramauro, C. Baiocchi, C. Medana, E. Montoneri and V. Boffa, J. Photochem. Photobiol., A, 2010, 209, 224–231 CrossRef CAS.
- A. Bianco Prevot, P. Avetta, D. Fabbri, E. Laurenti, T. Marchis, D. G. Perrone, E. Montoneri and V. Boffa, ChemSusChem, 2011, 4, 85–90 CrossRef CAS PubMed.
- L. K. Koopal, Y. Yang, A. J. Minnaard, P. L. M. Theunissen and W. H. Van Riemsdijk, Colloids Surf., A, 1998, 141, 385–395 CrossRef CAS.
- M. Klavins and L. Eglite, Colloids Surf., A, 2002, 203, 47–54 CrossRef CAS.
- D. Luo, Q. W. Yu, H. R. Yin and Y. Q. Feng, Anal. Chim. Acta, 2007, 588, 261–267 CrossRef CAS PubMed.
- P. Stathi and Y. Deligiannakis, J. Colloid Interface Sci., 2010, 351, 239–247 CrossRef CAS PubMed.
- J. Guczi, A. Angelova, R. A. Bulman and G. Szabo, React. Polym., 1992, 17, 61–68 CrossRef CAS.
- M. Klavins, L. Eglite and A. Zicmanis, Chemosphere, 2006, 62, 1500–1506 CrossRef CAS PubMed.
- F. Deganello, M. L. Tummino, C. Calabrese, M. L. Testa, P. Avetta, D. Fabbri, A. Bianco Prevot, E. Montoneri and G. Magnacca, New J. Chem., 2015, 39, 877–885 RSC.
- G. Pecchi, P. Reyes, I. Concha and J. L. G. Fierro, J. Catal., 1998, 179, 309–314 CrossRef CAS.
- A. R. Silva, K. Wilson, J. H. Clark and C. Freire, Microporous Mesoporous Mater., 2006, 91, 128–138 CrossRef CAS.
- M. Choi, W. Heo, F. Kleitz and R. Ryoo, Chem. Commun., 2003, 1340–1341 RSC.
- T. R. Pauly and T. J. Pinnavaia, Chem. Mater., 2001, 13, 987–993 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- M. L. Testa, V. La Parola, L. F. Liotta and A. M. Venezia, J. Mol. Catal. A: Chem., 2013, 367, 69–76 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin