Thermochemical recovery technology for improved modern engine fuel economy – part 1: analysis of a prototype exhaust gas fuel reformer
Abstract
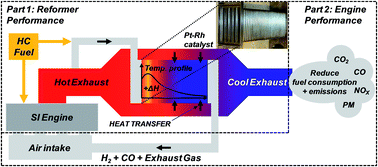
Exhaust gas fuel reforming has the potential to improve the thermal efficiency of internal combustion engines, as well as simultaneously reduce gaseous and particulate emissions. This thermochemical energy recovery technique aims to reclaim exhaust energy from the high temperature engine exhaust stream to drive catalytic endothermic fuel reforming reactions; these convert hydrocarbon fuel to hydrogen-rich reformate. The reformate is recycled back to the engine as Reformed Exhaust Gas Recirculation (REGR), which provides a source of hydrogen to enhance the engine combustion process and enable high levels of charge dilution; this process is especially promising for modern gasoline direct injection (GDI) engines. This paper presents a full-scale prototype gasoline reformer integrated with a multi-cylinder GDI engine. Performance is assessed in terms of the reformate composition, the temperature distribution across the catalyst, the reforming process (fuel conversion) efficiency and the amount of exhaust heat recovery achieved.

 Please wait while we load your content...
Please wait while we load your content...