Halogen-free ionic liquids: effect of chelated orthoborate anion structure on their lubrication properties†
Abstract
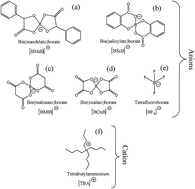
In transportation vehicles, a large portion of energy is consumed to overcome friction in the engine and associated components. An efficient lubricant system has a direct impact on saving energy and material loss by reducing the friction, wear, and corrosion. Herein, halogen-free chelated orthoborate ionic liquids were designed, synthesized and then evaluated as potential lubricant additives. The effect of the orthoborate anion structure on the thermal stability, corrosion, friction, and wear properties of ionic liquids were studied. The copper strip tests revealed the non-corrosiveness of bis(mandalato)borate (BMdB), bis(salicylato)borate (BScB), and bis(malonato)borate (BMlB) anion constituted ionic liquids. Whereas, the bis(oxalato)borate (BOxB) anion, eventually developed micro-pits of corrosion owing to its lower stability and acidic nature of the decomposed product. These ionic liquids as additives to the synthetic lube base oil significantly reduced both friction and wear. The degree of friction and wear reduction was influenced by the structure of associated anions. BMdB and BScB anion constituted ionic liquids exhibited excellent thermal stability, friction-reduction, and antiwear properties, which are attributed to their compact and chemically stable structure driven by higher intermolecular interactions and rigidity of aromatic rings. The chemical analysis of tribo-interfaces suggested the formation of an ionic liquid composed tribochemical product and that enhanced the lubrication properties. Being halogen-free, these ionic liquids could be energy efficient and environmentally-friendly substitutes to the conventional friction-reducing and antiwear additives.

 Please wait while we load your content...
Please wait while we load your content...