Carbon monolith with embedded mesopores and nanoparticles as a novel adsorbent for water treatment†
Abstract
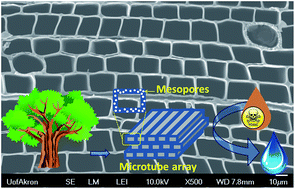
A novel carbon monolith material with embedded mesopores and nanoparticles has been synthesized via a facile catalytic graphitization process by using natural wood as the carbon precursor. BET results reveal a large specific surface area of 273.0 m2 g−1 and a narrow pore size distribution, with pore diameters of ∼4.0 nm. This unique material shows excellent toxic pollutants removal from water, including inorganic heavy metal ion (chromium(VI)) and organic dyes (methylene blue and methyl orange). Isothermal studies reveal its large removal capacities of 49.5, 61.7 and 68.2 mg g−1 for chromium(VI), methyl orange and methylene blue, respectively. Kinetic studies reveal that the removal process follows pseudo-second-order adsorption behavior. A mechanism study demonstrates that adsorption, electrostatic interaction and a redox reaction are involved in the pollutant removal process, and contribute to the large adsorption capacities and excellent rate performance. The widely accessible natural resources, low cost, convenient fabrication, and superior adsorption properties would facilitate this new material promising applications in the fields of water pollutant control and purification.

 Please wait while we load your content...
Please wait while we load your content...