The interaction between tannins and gliadin derived peptides in a celiac disease perspective†
Ricardo Dias,
Rosa Perez-Gregorio,
Nuno Mateus and
Victor De Freitas*
LAQV-REQUIMTE, Departamento de Química e Bioquímica, Faculdade de Ciências da Universidade do Porto, Rua do Campo Alegre 687, 4169-007 Porto, Portugal. E-mail: vfreitas@fc.up.pt
First published on 31st March 2015
Abstract
Given the high prevalence and lack of therapeutic means to treat celiac disease, the search for drugs and nutraceuticals that can block the initial stages of this chronic inflammatory disorder is a priority. Among the diversity of polyphenols, tannins have been described as the most reactive towards proline-rich proteins, which are structurally similar to gliadin peptides responsible for the onset of celiac disease. Therefore, the aim of this work was to verify the ability of different food tannins to interact with gliadin derived peptides, using fluorescence quenching and dynamic light scattering experiments. For that, a commercial raw extract of wheat gliadins was subjected to in vitro digestion followed by fractionation of the partially degraded peptides by semi-preparative HPLC. Each one of the seven collected mixtures were then characterized by ESI-MS/MS to identify their peptide composition. Using procyanidin B3, procyanidin trimers, procyanidin tetramers and an oligomeric mixture of high molecular weight procyanidins it was demonstrated, for the first time, that the association between those tannins and gliadin-derived peptides may occur, although in different contexts. Indeed, at the micromolar level it was observed by means of fluorescence assays that the size and structural features of the polyphenols is related to their quenching ability as a result of specific interactions or complex formation. At the millimolar level by using DLS, it was concluded that the procyanidins reactivity towards different peptide mixtures is mainly dependent on the peptide size with drastic effects on the dimension of the resulting aggregates. Overall, this study clearly opens new therapeutic perspectives for celiac disease, by using phenolic compounds as a nutraceutical approach to enhance the return of the full intestinal function in patients who show incomplete recovery in response to a gluten-free diet.
Introduction
Widely distributed in almost all plant foods and beverages, polyphenols are a group of natural compounds that are characterized by the presence of more than one phenolic unit per molecule.1 According to their structure, they are usually divided into hydrolysable tannins and phenylpropanoids, a subgroup that comprises lignins, flavonoids and condensed tannins. Overall the last years, several healthy effects have been attributed to the consumption of plant polyphenols as they provide significant protection against the development of several chronic diseases including cardiovascular and neurodegenerative diseases, cancer, diabetes, osteoporosis, infections, aging, asthma, etc.2 Recently, these versatile compounds proved to be effective in the inhibition of HIV as well of HSV.3,4 Besides these biological activities, some antinutritional effects have also been reported for dietary tannins due to their ability to complex specific proteins,5,6 namely digestive enzymes and salivary proteins.7 In particular, the aptitude of tannins to bind proteins in a specific and selective manner8 may further increase their potential applications in diverse knowledge fields as is the case of toxicology were tannin–protein complexes have been used as snake venom antidotes.9,10 Given the occurrence of several diseases caused by an inflammatory response to dietary proteins as well as the recognition that polyphenols may modulate the immune reactivity to those ones, the study of the interaction mechanism by which they potentially bind these proteins attain a great importance.11Celiac Disease (CD) is an acquired and prevalent food hypersensitivity disorder caused by permanent intolerance to ingested wheat gluten (mainly composed by gliadins and glutenins) and similar proteins of barley (secalins), rye (hordeins) and oats (avenins).12,13 Chronic inflammation of the small intestinal mucosa typically results in villous atrophy, crypt hyperplasia, dense lymphocytic infiltration and a variety of clinical symptoms that differ according to the age group.14–16 The principal toxic components of wheat gluten, the environmental stimuli responsible for both initiation and maintenance of the disease process have been identified and belong to a family of closely related proline and glutamine-rich proteins designated gliadins.17,18 These are mainly monomeric proteins with molecular weights around 28–55 kDa that are poorly digested in the human upper gastrointestinal tract.19,20 Despite the high prevalence and severe symptoms, presently, the only accepted treatment for celiac disease involves the strict dietary abstinence from these food grains.21 However, a complete avoidance of gluten is not easily achieved. It takes time, motivation and patience to become accustomed to such a diet. On the other hand, gluten-free products are not widely available and are usually more expensive than their gluten-containing counterparts.20,22 Among the diversity of polyphenols, tannins have been described as the most reactive towards proline-rich proteins (PRPs), which are structurally similar to celiac reactive peptides (CRPs).23,24 As these bioactive compounds present low intestinal absorption and suffer reduced metabolism in the human digestive system, they remain in the small intestine for extended periods of time,25 a feature that consents their interaction with gliadins and/or CRPs through essentially hydrophobic and hydrogen bonding.26 Therefore, tannins present a good potential as therapeutic agents for blocking the development of CD from both a nutraceutical and a pharmacologic point of view. Hence, the main goal of this study was to verify, for the first time, the ability of food tannins to interact with gliadin derived peptides, after their in vitro digestion.
Experimental
Reagents
All organic solvents used in this study were of analytical grade. Acetonitrile was purchased from Panreac while trifluoroacetic acid was obtained from Sigma-Aldrich. Pepsin from porcine gastric mucosa, pancreatin from porcine pancreas, α-chymotrypsin from bovine pancreas and gliadin from wheat were also acquired from Sigma-Aldrich.Grape seed tannin isolation
Condensed tannins were extracted from Vitis vinifera grape seeds and fractionated though a TSK Toyopearl HW-40(S) gel column (100 mm × 10 mm i.d., with 0.8 mL min−1 of methanol as eluent), according to the method described in the literature.27,28 Fraction II, obtained after elution with methanol/5% acetic acid (v/v) for about 14 h, contained mainly mono- and digalloylated procyanidin pentamers, hexamers and galloylated procyanidin heptamers, as determined by direct analysis through Electrospray Ionization Mass Spectrometry (ESI-MS) (Finnigan DECA XP PLUS).29 The mean molecular weight of that fraction (1524) was estimated based on the relative abundance of each flavanol present.Procyanidin B3, procyanidin trimer T1 and procyanidin tetramer TT1 synthesis
Procyanidin B3, procyanidin trimer T1 and procyanidin tetramer TT1 were obtained by hemisynthesis using (+)-taxifolin and (+)-catechin (ESI†).30,31 Following a TSK Toyopearl HW-40(S) gel column (300 mm × 10 mm i.d., with 0.8 mL min−1 of methanol as eluent), coupled to a UV-Vis detector (Gilson 115), several fractions were recovered and analyzed by ESI-MS (Finnigan DECA XP PLUS) yielding procyanidins with varying degrees of polymerization. The fractions containing procyanidin B3 ([M − H]− = 577), procyanidin trimer T1 ([M − H]− = 865) and procyanidin tetramer TT1 ([M − H]− = 1153) were isolated and freeze-dried. The purity of those fractions was assessed by LC-MS and direct MS analysis, and was higher than 95%.Separation and identification of gliadin derived peptides
A commercial raw extract of wheat gliadins was subjected to in vitro digestion in order to obtain gliadin derived peptides. A preliminary rough analysis of the complexity and protein composition in raw sample was previously studied by MALDI-TOF mass spectrometry (Fig. S1, in the ESI†).Peptide–tannin interaction assays
Statistical analysis
All assays were performed at least in n = 3 repetitions. Values are expressed as the arithmetic means ± SD. Statistical significance of the difference between various groups was evaluated by one-way analysis variance (ANOVA) followed by the Tuckey test. Differences were considered to be significant when P < 0.05. All statistical data were processed using the GraphPad Prism 5.0 (GraphPad Software, San Diego, USA).Results
Separation and characterization of wheat gliadin peptides
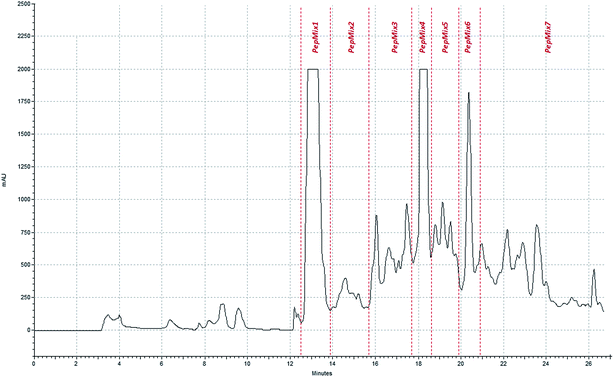
In order to study the ability of different tannins to interact with gliadin-derived peptides, seven peptidic fractions were isolated by semi-preparative HPLC after enzymatic hydrolysis of the wheat gliadins raw extract. Fig. 1 shows the acquired chromatographic profile of the simulated in vitro digestion. Each fraction was collected in different time intervals, as represented in the chromatogram. Thus, Pep Mix1 corresponded to the first eluted peak (∼13 min) while Pep Mix2 corresponded to the chromatographic zone ranging from 14 to 16 min. Pep Mix3 relates to the following region (16–18 min) and Pep Mix4 corresponded to the second major peak (∼18 min). Pep Mix5 matched the chromatographic zone comprised between the second and the third (Pep Mix6) major peaks, and finally, Pep Mix7 corresponded to the subsequent area ranging from 20 to 26 min. Each collected peptide mixture was then characterized by mass spectrometry in which the amino acid sequences of their main proteolytic products were determined based on those peptides fragmentation pattern. The comprehensive list of the best classified peptides is shown in Table S1 (in the ESI†). Indeed, these data confirm the high complexity of the initial digested raw sample that contained a large number of partially degraded fragments from the wheat gluten proteome. According to Table S1,† at least 33 different gluten proteins were identified. The identified peptides differed in both their amino acid composition and length (which was likely to increase with their elution order). Additionally, it was interesting to know that in Pep Mix4, 5, 6 and 7, certain gliadin-derived peptides contained some motifs associated with the induction of celiac disease. In that way, distinct patient-specific T cell epitopes such as ‘PFPQPQLPY’, ‘PQPQLPYPQ’, ‘QQPFPQQPQ’, ‘QQPQQPFPQ’ and ‘QQPQQPYPQ’ were identified in different peptides of the above mentioned mixtures (Table S1†).18,36 Although many of the identified peptides contained a considerable amount of Gln and Pro residues that may enhance an immune response in celiac patients,37 further studies are needed to clarify the immunological relevance of each collected peptide mixture.Fluorescence quenching studies
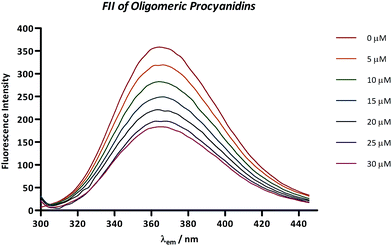
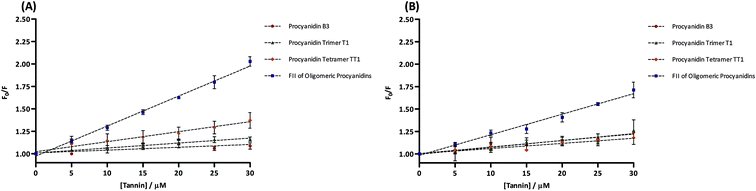
For fluorescence quenching measurements, only Pep Mix4 and Pep Mix6 were used since they were the ones who presented, in these experimental conditions, considerable fluorescence. Fig. 2 shows the fluorescence emission spectra obtained for Pep Mix4 with the addition of increasing concentrations of an oligomeric mixture of tannin procyanidins (FII). Independently of the tested tannin (including procyanidin B3, trimer T1 or tetramer TT1) it was observed that in all cases their addition caused a gradual decrease in the fluorescence intensity by quenching, without any significant shift in the emission maximum wavelength (data not shown). The same behavior was observed for Pep Mix6 (data not shown). The calculation of Ksv from Stern–Volmer plots (Fig. 3A for Pep Mix4 and Fig. 3B for Pep Mix6) demonstrated, mainly for Pep Mix4, that quenching depends on the polyphenolic structure, with fluorescence extinction being determined, in magnitude, by the procyanidins polymerization degree (Table 1). The Stern–Volmer constant (Ksv) is a measure of the ability of the tested polyphenols to interact with peptides in solution, reducing the fluorescence of the amino acid residue that is fluorescing (in this case tryptophan) and is determined as the slope of the F0/F = f([quencher]) plot, where F0 and F are the fluorescence intensities before and after the addition of the quencher. Oligomeric procyanidins of fraction II revealed the highest quenching constant in both peptide mixtures. On the other hand, there were no statistically significant differences between the quenching constants for procyanidin B3, procyanidin trimer T1 and procyanidin tetramer TT1 in Pep Mix6. In general, all the studied procyanidins appeared to be slightly more reactive towards the fourth peptide mixture than towards the sixth.| Ksv (M−1) | ||||
|---|---|---|---|---|
| Procyanidin B3 | Procyanidin trimer T1 | Procyanidin tetramer TT1 | FII of Oligomeric procyanidins | |
| Pep Mix4 | 3148 ± 841.3a | 5543 ± 428.2a | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 ± 1406b 150 ± 1406b |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 ± 906.5c 410 ± 906.5c |
| Pep Mix6 | 7660 ± 1502b | 7260 ± 946.8d | 5738 ± 695.7d | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 ± 1089e 890 ± 1089e |
Light scattering studies
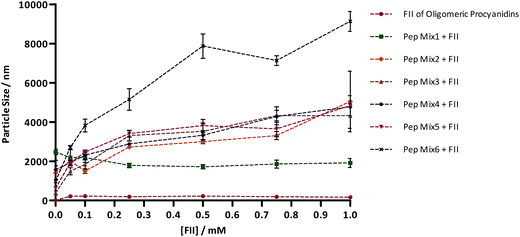
Light scattering measurements were developed in order to characterize the size of the different aggregates formed between the digested peptides and an oligomeric mixture of tannin procyanidins (FII) (Fig. 4). According to DLS, Pep Mix6 produced the larger aggregates, being this behavior observed across the whole range of tannin concentration. Still, by visual examination, it was found that the addition of increasing concentrations of procyanidins to Pep Mix7 resulted in immediate cloudiness and subsequent precipitation of insoluble aggregates in the DLS cell. As an unusual extensive aggregation took place in the latter mixture, it became virtually impossible to correctly measure the aggregates size by such a light scattering study, with the obtained values exhibiting a tremendous variability (data not shown). Pep Mix1 seems to be the less reactive towards oligomeric procyanidins since the dimension of the resulting aggregates, if any, remained nearly unchanged. For the intermediate peptide mixtures (Pep Mix2 to Pep Mix5), no significant differences were detected in the size of the formed aggregates, all of them having a very similar behavior in the whole range of procyanidin FII concentration.Discussion
The interaction between procyanidins and peptide mixtures obtained after in vitro digestion of wheat gliadins was conducted using two different techniques to cover a large range of concentrations: from the micromolar range with fluorescence to the millimolar range with DLS. Although it has been tested the possibility of using the same tannin concentration range in the fluorescence quenching measurements and DLS assays, this proved to be virtually impossible given the substantial differences in the sensitivity and detection limit of those two techniques.To interpret the data from fluorescence quenching studies, it is important to understand what kind of interactions take place between the quencher and the fluorophore. As represented in Fig. 3A and B, for both peptide mixtures and procyanidins tested, the respective Stern–Volmer plots were all linear, which means that only one type of quenching occurred (dynamic or static). In the case of a dynamic mechanism for fluorescence quenching, it is the diffusion-limited collision between the quencher and the fluorophore molecules that allows the energy transfer without radiation. Quenching can also be caused by the formation of a complex between those two compounds that does not fluoresce after returning from the excited state – static quenching.38 To verify if that quenching is due to a specific interaction, or complex formation, it is essential to calculate the bimolecular quenching constant (kq), dividing the obtained Ksv values by the lifetime of each peptide mixture in the absence of the quencher (τ0). The dynamic mechanism (diffusion-limited quenching) typically results in values of kq near 1010 M−1 s−1.39 Since all the obtained values for kq were more than 90-fold higher, this suggests that the interaction of procyanidins with both peptide mixtures involved the formation of a stable complex (Table 2).40,41 In vivo, this kind of interaction could eventually predict the potential of procyanidins to interfere with the availability of celiac reactive peptides, blocking their immunological and toxic effects on the intestinal mucosa. On the other hand, this hypothetical ability of procyanidins to snatch such peptides appears to be highly dependent on its structure, or more specifically its degree of polymerization. In fact, assuming that the Stern–Volmer quenching constant corresponds to a binding constant, this trend is well confirmed in Fig. 3A, where its value increased from procyanidin B3 to FII. The essence of such behavior is based on the fact that the number of catechin units and galloyl groups increases with the molecular weight of the procyanidins. This results in a higher number of aromatic rings and hydroxyl groups that may be involved in hydrophobic and hydrogen bonding with several protein binding sites.30 Therefore, a stronger binding affinity was anticipated for the high molecular weight procyanidin oligomers, as is the case of FII. For Pep Mix6, however, this behavior does not appear to be as linear as previously described, since the constants of procyanidin B3, procyanidin trimer T1 and procyanidin tetramer TT1 showed a similar magnitude (statistically, they are not significantly different) (Table 1). The differences between the bindings of the same polyphenol to Pep Mix4 and Pep Mix6 may reflect structural differences between those two peptide mixtures, including the amino acid composition of the peptides that were involved in complex formation.42,43
| τ0 (s) | kq × 10−12 (M−1 s−1) | ||||
|---|---|---|---|---|---|
| Procyanidin B3 | Procyanidin trimer T1 | Procyanidin tetramer TT1 | FII of Oligomeric procyanidins | ||
| Pep Mix4 | 3.509 × 10−9 | 0.897 ± 0.240a | 1.580 ± 0.122a | 3.177 ± 0.401b | 9.521 ± 0.258c |
| Pep Mix6 | 3.302 × 10−9 | 2.320 ± 0.455d | 2.199 ± 0.287d | 1.738 ± 0.211d | 6.932 ± 0.330e |
By DLS (Fig. 4), the results are somewhat opposed to the ones obtained by fluorescence quenching in that the FII of oligomeric procyanidins appeared to be slightly more reactive towards Pep Mix6. The reason for this may be explained based on differences that are inherent to these two techniques. On one hand, fluorescence quenching is an extremely sensitive and selective approach that gives information about the molecular environment in the vicinity of a chromophore molecule. On the other hand, DLS is less selective than fluorescence quenching assays giving information about the size of structures in solution at a supramolecular level. In addition, it is important to highlight that the specificity of tannin–protein interaction, among other things, is strongly dependent on the protein and tannin concentration range.44,45 So, while in dilute solutions the tannins may specifically bind to individual peptides in a way that is essentially determined by both structural features and without any protein aggregation, when the tannin–peptide molar ratio exceeds a threshold, the aggregation of peptides may occur with tannins bridging them together.46 Since this event is highly favored by the complexity of the peptides available to interaction, and because the number of peptides with increasing size increased from Pep Mix1 to Pep Mix7, it was assumed, for the peptide/procyanidin concentration range used in DLS, that the size factor may become a much more decisive driving force when determining the dimension of the resulting aggregates. Consequently, peptide mixtures collected later by semi-preparative HPLC produced the largest aggregates eventually leading to their precipitation when the transfer of nonaggregated peptides to the aggregates became too pronounced.
Although the main goal herein was to demonstrate, for the first time, the potential of different tannins to interact with peptides resulting from incomplete degradation of gliadins, a much deeper approach will be necessary to differentiate those products that are indeed important from a disease point of view and also to specifically study their higher or lower propensity to react with food tannins. Remaining unclear how the association process described herein does interfere with those peptides immunogenicity and ability to induce damage in the intestinal mucosa, the finding that some of them contains specific-T cell epitopes associated to celiac disease (Table S1, in the ESI†) creates high expectations for the following studies, aiming at further evaluate the potential protective effect of tannins on cytotoxicity of gluten peptides.
Conclusions
The interaction of gliadin-derived peptide mixtures, characterized by proteomic approach, with different procyanidins was evaluated by the measurement of the intrinsic fluorescence intensity of tryptophan residues and the aggregates size was further studied using dynamic light scattering (DLS). In general, both techniques allowed to prove and evaluate the binding affinity between those elements, although in different contexts. On the one hand, fluorescence quenching measurements demonstrated, at the micromolar level, that the size and structural features of the polyphenols is related to their quenching ability. So, for the same peptide mixture, the smaller procyanidin (B3) was the weakest quenching molecule because it was the one that provided fewer binding groups. However, in different peptide mixtures (Pep Mix4 vs. Pep Mix6), the same polyphenolic molecule could have different binding affinities, which is probably related to the differential amino acid composition of the respective peptides. At the milimolar level, dynamic light scattering measurements demonstrated that for a higher peptide–tannin concentration range, the procyanidins reactivity towards different peptide mixtures is mainly dependent on those peptides size. Overall, this study clearly opens new therapeutical perspectives for celiac disease by using phenolic compounds as a nutraceutical approach for modulation of this chronic inflammatory condition. The next steps will require further biological studies involving these peptides in the presence of different polyphenols to assess the physiological and biochemical consequences of the association process described herein.Acknowledgements
We thank the Fundaçao para a Ciencia e Tecnologia (FCT) which funded this work by one postdoctoral fellowship (SFRH/BPD/85293/2012) and by a research project grant (NORTE-07-0162-FEDER-000048).Notes and references
- J. Serrano, R. Puupponen-Pimiä, A. Dauer, A.-M. Aura and F. Saura-Calixto, Mol. Nutr. Food Res., 2009, 53, S310–S329 Search PubMed.
- K. B. Pandey and S. I. Rizvi, Oxid. Med. Cell. Longevity, 2009, 2, 270–278 CrossRef PubMed.
- M. Helfer, H. Koppensteiner, M. Schneider, S. Rebensburg, S. Forcisi, C. Mueller, P. Schmitt-Kopplin, M. Schindler and R. Brack-Werner, PLoS One, 2014, 9(1), e87487 Search PubMed.
- X. Qiu, M. Zhong, Y. Xiang, C. Qu, Y. Pei, Y. Zhang, C. Yang, J. Gasteiger, J. Xu, Z. Liu and Y. Wang, Med. Chem., 2014, 10, 388–401 CrossRef CAS.
- S. Soares, N. Mateus and V. de Freitas, Food Res. Int., 2012, 49, 807–813 CrossRef CAS PubMed.
- T. Ozdal, E. Capanoglu and F. Altay, Food Res. Int., 2013, 51, 954–970 CrossRef CAS PubMed.
- M. He, H. Tian, X. Luo, X. Qi and X. Chen, Molecules, 2015, 20, 1434–1451 CrossRef CAS PubMed.
- M. R. Perez-Gregorio, N. Mateus and V. de Freitas, Langmuir, 2014, 30, 8528–8537 CrossRef CAS PubMed.
- B. Prabha and N. Savithramma, Int. J. Pharm. Pharm. Sci., 2014, 6(8), 180–182 Search PubMed.
- V. M. de Moura, L. A. Freitas de Sousa, M. Cristina Dos-Santos, J. D. Almeida Raposo, A. Evangelista Lima, R. B. de Oliveira, M. N. da Silva and R. H. Veras Mourao, J. Ethnopharmacol., 2015, 161, 224–232 CrossRef PubMed.
- J. Ognjenovic, M. Stojadinovic, M. Milcic, D. Apostolovic, J. Vesic, I. Stambolic, M. Atanaskovic-Markovic, M. Simonovic and T. C. Velickovic, Food Chem., 2014, 164, 36–43 CrossRef CAS PubMed.
- L. M. Sollid, Nat. Rev. Immunol., 2002, 2, 647–655 CrossRef CAS PubMed.
- H. Arentz-Hansen, B. Fleckenstein, O. Molberg, H. Scott, F. Koning, G. Jung, P. Roepstorff, K. E. A. Lundin and L. M. Sollid, PLoS Med., 2004, 1, 84–92 CrossRef CAS PubMed.
- G. Ferretti, T. Bacchetti, S. Masciangelo and L. Saturni, Nutrients, 2012, 4, 243–257 CrossRef CAS PubMed.
- D. A. van Heel and J. West, Gut, 2006, 55, 1037–1046 CrossRef CAS PubMed.
- A. Fasano and C. Catassi, Gastroenterology, 2001, 120, 636–651 CrossRef CAS PubMed.
- L. Shan, O. Molberg, I. Parrot, F. Hausch, F. Filiz, G. M. Gray, L. M. Sollid and C. Khosla, Science, 2002, 297, 2275–2279 CrossRef CAS PubMed.
- L. Shan, S. W. Qiao, H. Arentz-Hansen, O. Molberg, G. M. Gray, L. M. Sollid and C. Khosla, J. Proteome Res., 2005, 4, 1732–1741 CrossRef CAS PubMed.
- H. Wieser, Food Microbiol., 2007, 24, 115–119 CrossRef CAS PubMed.
- P. H. R. Green and C. Cellier, N. Engl. J. Med., 2007, 357, 1731–1743 CrossRef CAS PubMed.
- P. C. Calder, R. Albers, J. M. Antoine, S. Blum, R. Bourdet-Sicard, G. A. Ferns, G. Folkerts, P. S. Friedmann, G. S. Frost, F. Guarner, M. Lovik, S. Macfarlane, P. D. Meyer, L. M'Rabet, M. Serafini, W. van Eden, J. van Loo, W. V. Dias, S. Vidry, B. M. Winklhofer-Roob and J. Zhao, Br. J. Nutr., 2009, 101, S1–S45 Search PubMed.
- L. M. Sollid and C. Khosla, Nat. Clin. Pract. Gastroenterol. Hepatol., 2005, 2, 140–147 CrossRef CAS PubMed.
- B. G. Green, Acta Psychol., 1993, 84, 119–125 CrossRef CAS.
- S. Soares, R. Vitorino, H. Osorio, A. Fernandes, A. Venancio, N. Mateus, F. Amado and V. de Freitas, J. Agric. Food Chem., 2011, 59, 5535–5547 CrossRef CAS PubMed.
- C. Santos-Buelga and A. Scalbert, J. Sci. Food Agric., 2000, 80, 1094–1117 CrossRef CAS.
- V. de Freitas and N. Mateus, Curr. Org. Chem., 2012, 16, 724–746 CrossRef CAS.
- V. A. P. de Freitas, Y. Glories, G. Bourgeois and C. Vitry, Phytochemistry, 1998, 49, 1435–1441 CrossRef CAS.
- V. A. P. de Freitas, Y. Glories and M. Laguerre, J. Agric. Food Chem., 1998, 46, 376–382 CrossRef CAS PubMed.
- S. Gonzalez-Manzano, N. Mateus, V. de Freitas and C. Santos-Buelga, Eur. Food Res. Technol., 2008, 227, 83–92 CrossRef CAS.
- T. A. Geissman and N. n. Yoshimur, Tetrahedron Lett., 1966, 2669–2673 CrossRef CAS.
- J. A. Delcour, D. Ferreira and D. G. Roux, J. Chem. Soc., Perkin Trans. 1, 1983, 1711–1717 RSC.
- A. Papadopoulou, R. J. Green and R. A. Frazier, J. Agric. Food Chem., 2005, 53, 158–163 CrossRef CAS PubMed.
- S. Soares, N. Mateus and V. De Freitas, J. Agric. Food Chem., 2007, 55, 6726–6735 CrossRef CAS PubMed.
- R. Goncalves, N. Mateus, I. Pianet, M. Laguerre and V. de Freitas, Langmuir, 2011, 27, 13122–13129 CrossRef CAS PubMed.
- M. Ferreira and P. Gameiro, J. Membr. Biol., 2015, 248, 125–136 CrossRef CAS PubMed.
- C. Osorio, N. Wen, R. Gemini, R. Zemetra, D. von Wettstein and S. Rustgi, Funct. Integr. Genomics, 2012, 12, 417–438 CrossRef CAS PubMed.
- A. Real, I. Comino, M. de Lourdes Moreno, M. Angel Lopez-Casado, P. Lorite, M. Isabel Torres, A. Cebolla and C. Sousa, PLoS One, 2014, 9(6), e100917 Search PubMed.
- R. Goncalves, N. Mateus and V. De Freitas, J. Agric. Food Chem., 2010, 58, 11924–11931 CrossRef CAS PubMed.
- J. R. Lackowicz, in Principles of Fluorescence Spectroscopy, Kluwer Academic/Plenum Publishers, New York, 2nd edn, 1999 Search PubMed.
- R. Goncalves, N. Mateus and V. de Freitas, Food Chem., 2011, 125, 665–672 CrossRef CAS PubMed.
- F. Rasoulzadeh, H. N. Jabary, A. Naseri and M.-R. Rashidi, Spectrochim. Acta, Part A, 2009, 72, 190–193 CrossRef PubMed.
- A. E. Hagerman and L. G. Butler, J. Biol. Chem., 1981, 256, 4494–4497 CAS.
- A. J. Charlton, N. J. Baxter, M. L. Khan, A. J. G. Moir, E. Haslam, A. P. Davies and M. P. Williamson, J. Agric. Food Chem., 2002, 50, 1593–1601 CrossRef CAS PubMed.
- V. de Freitas and N. Mateus, J. Agric. Food Chem., 2001, 49, 940–945 CrossRef CAS PubMed.
- L. G. Butler, D. J. Riedl, D. G. Lebryk and H. J. Blytt, J. Am. Oil Chem. Soc., 1984, 61, 916–920 CrossRef CAS.
- F. Canon, F. Pate, V. Cheynier, P. Sarni-Manchado, A. Giuliani, J. Perez, D. Durand, J. Li and B. Cabane, Langmuir, 2013, 29, 1926–1937 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: It includes detailed information related to the experimental section (procyanidin B3, procyanidin trimer T1 and procyanidin tetramer TT1 synthesis, MALDI-TOF mass spectrometry analysis of gliadin raw extract, in vitro digestion of gliadin raw extract and fluorescence lifetimes determination) as well as additional results (Fig. S1 and Table S1). See DOI: 10.1039/c5ra02968f |
| This journal is © The Royal Society of Chemistry 2015 |